A Portable Microfluidic System for Point-of-Care Detection of Multiple Protein Biomarkers
- PMID: 33804983
- PMCID: PMC8063924
- DOI: 10.3390/mi12040347
A Portable Microfluidic System for Point-of-Care Detection of Multiple Protein Biomarkers
Abstract
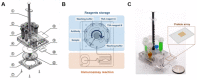
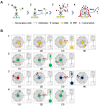
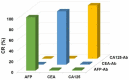
Protein biomarkers are indicators of many diseases and are commonly used for disease diagnosis and prognosis prediction in the clinic. The urgent need for point-of-care (POC) detection of protein biomarkers has promoted the development of automated and fully sealed immunoassay platforms. In this study, a portable microfluidic system was established for the POC detection of multiple protein biomarkers by combining a protein microarray for a multiplex immunoassay and a microfluidic cassette for reagent storage and liquid manipulation. The entire procedure for the immunoassay was automatically conducted, which included the antibody-antigen reaction, washing and detection. Alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA) and carcinoma antigen 125 (CA125) were simultaneously detected in this system within 40 min with limits of detection of 0.303 ng/mL, 1.870 ng/mL, and 18.617 U/mL, respectively. Five clinical samples were collected and tested, and the results show good correlations compared to those measured by the commercial instrument in the hospital. The immunoassay cassette system can function as a versatile platform for the rapid and sensitive multiplexed detection of biomarkers; therefore, it has great potential for POC diagnostics.
Keywords: immunoassay; microarray; microfluidic cassette; multiplex measurement; point-of-care testing; protein biomarker.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Biomarkers Definitions Working Group. Atkinson A.J., Jr., Colburn W.A., DeGruttola V.G., DeMets D.L., Downing G.J., Hoth D.F., Oates J.A., Peck C.C., Schooley R.T., et al. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001;69:89–95. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

