Immunomodulatory and Anticancer Activities of Hyacinthus orientalis L.: An In Vitro and In Vivo Study
- PMID: 33805000
- PMCID: PMC8063964
- DOI: 10.3390/plants10040617
Immunomodulatory and Anticancer Activities of Hyacinthus orientalis L.: An In Vitro and In Vivo Study
Abstract
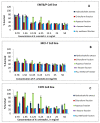
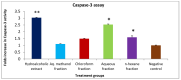
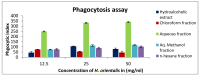
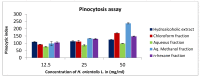
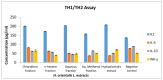
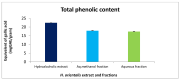
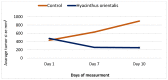
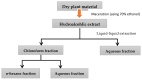
Hyacinthus orientalis L. (family Hyacinthaceae) is traditionally used to treat different diseases including cancer. In this study, the anticancer and immunomodulatory effects of this plant were evaluated. Hydroalcoholic extract was prepared, and different solvent fractions were obtained using solvent-solvent extraction. In the anticancer part, MTT assay and caspase-3 ELISA kits were used to measure the antiproliferative and apoptosis induction ability for each extract, respectively. In the immunomodulatory part, lymphocyte proliferation assay and cytokines detection kit were used to measure the effect of extracts of acquired immunity. Phagocytosis and pinocytosis induction were used to evaluate the effect of extracts on the innate immunity. GC-MS, LC-MS, and Foline-Ciocalteu assays were used to identify the chemical composition of the plant. Balb/C mice were inoculated with breast cancer and treated with hydroalcoholic extract of H. orientalis L. Results showed that hydroalcoholic extract and n-hexane fraction were highly effective in apoptosis induction. Both extract and fraction were also effective in stimulating lymphocytes proliferation and phagocytosis. Significant reduction in tumor size was achieved after treating tumor-bearing mice with hydroalcoholic extract. Additionally, high cure percentages (50%) were obtained in treated mice. Results of this study showed that H. orientalis L. has promising anticancer and immunomodulatory activities. However, further studies are needed to explore more details of apoptosis induction ability and other mechanisms of action and to measure different signaling pathways responsible for the anticancer and immunomodulatory response.
Keywords: anticancer; apoptosis; immunomodulatory; plant extracts; traditional medicine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Immunomodulatory and Anticancer Activities of Barley Bran Grown in Jordan: An in vitro and in vivo Study.Front Nutr. 2022 May 18;9:838373. doi: 10.3389/fnut.2022.838373. eCollection 2022. Front Nutr. 2022. PMID: 35662936 Free PMC article.
-
Xanthium spinosum L. Extracts Inhibit Breast Cancer in Mice by Apoptosis Induction and Immune System Modulation.Pharmaceuticals (Basel). 2022 Dec 2;15(12):1504. doi: 10.3390/ph15121504. Pharmaceuticals (Basel). 2022. PMID: 36558955 Free PMC article.
-
Terfezia boudieri: A Desert Truffle With Anticancer and Immunomodulatory Activities.Front Nutr. 2020 Apr 8;7:38. doi: 10.3389/fnut.2020.00038. eCollection 2020. Front Nutr. 2020. PMID: 32322585 Free PMC article.
-
Immunomodulatory, Anticancer, and Antimicrobial Effects of Rice Bran Grown in Iraq: An In Vitro and In Vivo Study.Pharmaceuticals (Basel). 2022 Dec 1;15(12):1502. doi: 10.3390/ph15121502. Pharmaceuticals (Basel). 2022. PMID: 36558953 Free PMC article.
-
Antioxidant and Antiproliferation Activities of Lemon Verbena (Aloysia citrodora): An In Vitro and In Vivo Study.Plants (Basel). 2022 Mar 16;11(6):785. doi: 10.3390/plants11060785. Plants (Basel). 2022. PMID: 35336667 Free PMC article.
Cited by
-
Anticancer, Immunomodulatory, and Phytochemical Screening of Carthamus oxyacantha M.Bieb Growing in the North of Iraq.Plants (Basel). 2023 Dec 22;13(1):42. doi: 10.3390/plants13010042. Plants (Basel). 2023. PMID: 38202350 Free PMC article.
-
Antiproliferative Effects of the Methanolic Petiole Extract of Eichhornia crassipes Against Sloan Kettering Melanoma 5 Cell Line: An In Vitro Study.Cureus. 2022 Oct 21;14(10):e30554. doi: 10.7759/cureus.30554. eCollection 2022 Oct. Cureus. 2022. PMID: 36415425 Free PMC article.
-
In Vitro Biological Activity and Lymphoma Cell Growth Inhibition by Selected Mexican Medicinal Plants.Life (Basel). 2023 Apr 6;13(4):958. doi: 10.3390/life13040958. Life (Basel). 2023. PMID: 37109486 Free PMC article.
-
Immunomodulatory and Anticancer Activities of Barley Bran Grown in Jordan: An in vitro and in vivo Study.Front Nutr. 2022 May 18;9:838373. doi: 10.3389/fnut.2022.838373. eCollection 2022. Front Nutr. 2022. PMID: 35662936 Free PMC article.
-
Phytol from Scoparia dulcis prevents NF-κB-mediated inflammatory responses during macrophage polarization.3 Biotech. 2024 Mar;14(3):80. doi: 10.1007/s13205-024-03924-9. Epub 2024 Feb 17. 3 Biotech. 2024. PMID: 38375513 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

