Advanced Techniques in the Percutaneous Ablation of Liver Tumours
- PMID: 33805107
- PMCID: PMC8064108
- DOI: 10.3390/diagnostics11040585
Advanced Techniques in the Percutaneous Ablation of Liver Tumours
Abstract
Percutaneous ablation is an accepted treatment modality for primary hepatocellular carcinoma (HCC) and liver metastases. The goal of curative ablation is to cause the necrosis of all tumour cells with an adequate margin, akin to surgical resection, while minimising local damage to non-target tissue. Aside from the ablative modality, the proceduralist must decide the most appropriate imaging modality for visualising the tumour and monitoring the ablation zone. The proceduralist may also employ protective measures to minimise injury to non-target organs. This review article discusses the important considerations an interventionalist needs to consider when performing the percutaneous ablation of liver tumours. It covers the different ablative modalities, image guidance, and protective techniques, with an emphasis on new and advanced ablative modalities and adjunctive techniques to optimise results and achieve satisfactory ablation margins.
Keywords: hepatocellular carcinoma; liver metastases; percutaneous ablation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



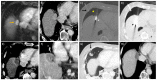

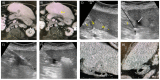
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

