CXCR4-Directed PET/CT in Patients with Newly Diagnosed Neuroendocrine Carcinomas
- PMID: 33805264
- PMCID: PMC8067200
- DOI: 10.3390/diagnostics11040605
CXCR4-Directed PET/CT in Patients with Newly Diagnosed Neuroendocrine Carcinomas
Abstract
We aimed to elucidate the diagnostic potential of the C-X-C motif chemokine receptor 4 (CXCR4)-directed positron emission tomography (PET) tracer 68Ga-Pentixafor in patients with poorly differentiated neuroendocrine carcinomas (NEC), relative to the established reference standard 18F-FDG PET/computed tomography (CT). In our database, we retrospectively identified 11 treatment-naïve patients with histologically proven NEC, who underwent 18F-FDG and CXCR4-directed PET/CT for staging and therapy planning. The images were analyzed on a per-patient and per-lesion basis and compared to immunohistochemical staining (IHC) of CXCR4 from PET-guided biopsies. 68Ga-Pentixafor visualized tumor lesions in 10/11 subjects, while18F-FDG revealed sites of disease in all 11 patients. Although weak to moderate CXCR4 expression could be corroborated by IHC in 10/11 cases, 18F-FDG PET/CT detected significantly more tumor lesions (102 vs. 42; total lesions, n = 107; p < 0.001). Semi-quantitative analysis revealed markedly higher 18F-FDG uptake as compared to 68Ga-Pentixafor (maximum and mean standardized uptake values (SUV) and tumor-to-background ratios (TBR) of cancerous lesions, SUVmax: 12.8 ± 9.8 vs. 5.2 ± 3.7; SUVmean: 7.4 ± 5.4 vs. 3.1 ± 3.2, p < 0.001; and, TBR 7.2 ± 7.9 vs. 3.4 ± 3.0, p < 0.001). Non-invasive imaging of CXCR4 expression in NEC is inferior to the reference standard 18F-FDG PET/CT.
Keywords: 18F-FDG; 68Ga-Pentixafor; CXCR4; NEC; NET.
Conflict of interest statement
H.J.W. is founder and shareholder of Scintomics. All other authors declare no conflicts of interest. “The authors declare no conflict of interest.” The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
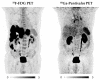
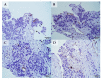
References
-
- Strosberg J., Wolin E., Chasen B., Kulke M., Bushnell D., Caplin M., Baum R.P., Kunz P., Hobday T., Hendifar A., et al. Health-Related Quality of Life in Patients With Progressive Midgut Neuroendocrine Tumors Treated With (177)Lu-Dotatate in the Phase III NETTER-1 Trial. J. Clin. Oncol. 2018;36:2578–2584. doi: 10.1200/JCO.2018.78.5865. - DOI - PMC - PubMed
-
- Baum R.P., Kluge A.W., Kulkarni H., Schorr-Neufing U., Niepsch K., Bitterlich N., Van Echteld C.J. [(177)Lu-DOTA](0)-D-Phe(1)-Tyr(3)-Octreotide ((177)Lu-DOTATOC) For Peptide Receptor Radiotherapy in Patients with Advanced Neuroendocrine Tumours: A Phase-II Study. Theranostics. 2016;6:501–510. doi: 10.7150/thno.13702. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

