A New Era in Engineering Plastics: Compatibility and Perspectives of Sustainable Alipharomatic Poly(ethylene terephthalate)/Poly(ethylene 2,5-furandicarboxylate) Blends
- PMID: 33805314
- PMCID: PMC8038036
- DOI: 10.3390/polym13071070
A New Era in Engineering Plastics: Compatibility and Perspectives of Sustainable Alipharomatic Poly(ethylene terephthalate)/Poly(ethylene 2,5-furandicarboxylate) Blends
Abstract
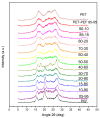
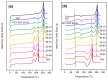
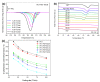
The industrialisation of poly(ethylene 2,5-furandicarboxylate) for total replacement of poly(ethylene terephthalate) in the polyester market is under question. Preparation of high-performing polymer blends is a well-established strategy for tuning the properties of certain homopolymers and create tailor-made materials to meet the demands for a number of applications. In this work, the structure, thermal properties and the miscibility of a series of poly(ethylene terephthalate)/poly(ethylene 2,5-furandicarboxylate) (PET/PEF) blends have been studied. A number of thermal treatments were followed in order to examine the thermal transitions, their dynamic state and the miscibility characteristics for each blend composition. Based on their glass transition temperatures and melting behaviour the PET/PEF blends are miscible at high and low poly(ethylene terephthalate) (PET) contents, while partial miscibility was observed at intermediate compositions. The multiple melting was studied and their melting point depression was analysed with the Flory-Huggins theory. In an attempt to further improve miscibility, reactive blending was also investigated.
Keywords: blends; crystallization; poly(ethylene furanoate); poly(ethylene terephthalate).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
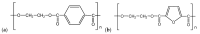







References
-
- Sanders J.P.M., Clark J.H., Harmsen G.J., Heeres H.J., Heijnen J.J., Kersten S.R.A., Swaaij W.P.M., Moulijin J.A. Process intensification in the future production of base chemicals from biomass. Chem. Eng. Process. 2012;51:117–136. doi: 10.1016/j.cep.2011.08.007. - DOI
-
- Zhang L., Jiang Y., Xiong Z., Liu X., Na H., Zhang R., Zhu J. Highly recoverable rosin-based shape memory polyurethanes. J. Mater. Chem. A. 2013;1:3263–3267. doi: 10.1039/c3ta01655b. - DOI
-
- Storz H., Vorlop K.D. Bio-based plastics: Status, challenges and trends. Appl. Agric. For. Res. 2013;63:321–332.
-
- Mülhaupt R. Green Polymer Chemistry and Bio-based Plastics: Dreams and Reality. Macromol. Chem. Phys. 2012;214:159–174. doi: 10.1002/macp.201200439. - DOI
-
- Schneiderman D.K., Hillmyer M.A. 50th Anniversary Perspective: There Is a Great Future in Sustainable Polymers. Macromolecules. 2017;50:3733–3749. doi: 10.1021/acs.macromol.7b00293. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

