Mechanisms of Cisplatin-Induced Acute Kidney Injury: Pathological Mechanisms, Pharmacological Interventions, and Genetic Mitigations
- PMID: 33805488
- PMCID: PMC8036620
- DOI: 10.3390/cancers13071572
Mechanisms of Cisplatin-Induced Acute Kidney Injury: Pathological Mechanisms, Pharmacological Interventions, and Genetic Mitigations
Abstract
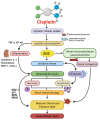
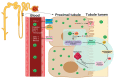
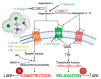
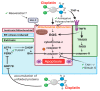
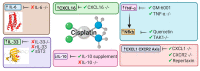
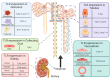
Administration of the chemotherapeutic agent cisplatin leads to acute kidney injury (AKI). Cisplatin-induced AKI (CIAKI) has a complex pathophysiological map, which has been linked to cellular uptake and efflux, apoptosis, vascular injury, oxidative and endoplasmic reticulum stress, and inflammation. Despite research efforts, pharmaceutical interventions, and clinical trials spanning over several decades, a consistent and stable pharmacological treatment option to reduce AKI in patients receiving cisplatin remains unavailable. This has been predominately linked to the incomplete understanding of CIAKI pathophysiology and molecular mechanisms involved. Herein, we detail the extensively known pathophysiology of cisplatin-induced nephrotoxicity that manifests and the variety of pharmacological and genetic alteration studies that target them.
Keywords: AKI; acute kidney injury; cisplatin; cisplatin-induced acute kidney injury; nephrotoxicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Li Z., Zhang P., Ma Q., Wang D., Zhou T. Cisplatin-based chemoradiotherapy with 5-fluorouracil or pemetrexed in patients with locally advanced, unresectable esophageal squamous cell carcinoma: A retrospective analysis. Mol. Clin. Oncol. 2017;6:743–747. doi: 10.3892/mco.2017.1222. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

