Biomedical Applications of Bacteria-Derived Polymers
- PMID: 33805506
- PMCID: PMC8036740
- DOI: 10.3390/polym13071081
Biomedical Applications of Bacteria-Derived Polymers
Abstract
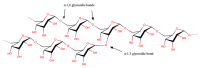
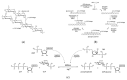
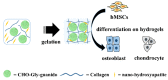
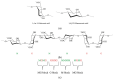
Plastics have found widespread use in the fields of cosmetic, engineering, and medical sciences due to their wide-ranging mechanical and physical properties, as well as suitability in biomedical applications. However, in the light of the environmental cost of further upscaling current methods of synthesizing many plastics, work has recently focused on the manufacture of these polymers using biological methods (often bacterial fermentation), which brings with them the advantages of both low temperature synthesis and a reduced reliance on potentially toxic and non-eco-friendly compounds. This can be seen as a boon in the biomaterials industry, where there is a need for highly bespoke, biocompatible, processable polymers with unique biological properties, for the regeneration and replacement of a large number of tissue types, following disease. However, barriers still remain to the mass-production of some of these polymers, necessitating new research. This review attempts a critical analysis of the contemporary literature concerning the use of a number of bacteria-derived polymers in the context of biomedical applications, including the biosynthetic pathways and organisms involved, as well as the challenges surrounding their mass production. This review will also consider the unique properties of these bacteria-derived polymers, contributing to bioactivity, including antibacterial properties, oxygen permittivity, and properties pertaining to cell adhesion, proliferation, and differentiation. Finally, the review will select notable examples in literature to indicate future directions, should the aforementioned barriers be addressed, as well as improvements to current bacterial fermentation methods that could help to address these barriers.
Keywords: bacteria; biodegradable polymers; biomaterial; biopolymer; biosynthesis; drug delivery; hydrogel; polymer science; regenerative medicine; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
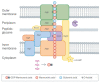
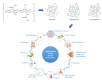
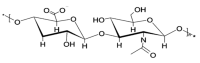
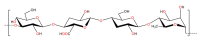
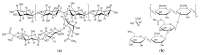
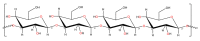
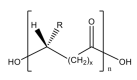
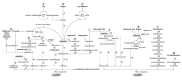
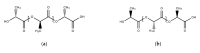
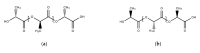
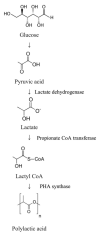
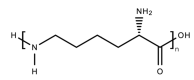

References
-
- PlasticsEurope Plastics—The Facts 2019: An Analysis of European Plastics Production, Demand and Waste Data. Belgium. [(accessed on 15 October 2020)];2019 Available online: https://www.plasticseurope.org/application/files/9715/7129/9584/FINAL_we....
-
- Meikle J.L. American Plastic: A Cultural History. Rutgers University Press; New Brunswick, NJ, USA: 1995.
-
- Potter K.D. The early history of the resin transfer moulding process for aerospace applications. Compos. Part A Appl. Sci. Manuf. 1999;30:619–621. doi: 10.1016/S1359-835X(98)00179-1. - DOI
-
- Rea S., Bonfield W. Biocomposites for medical applications. J. Australas. Ceram. Soc. 2004;40:43–57.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

