A High-Accuracy Model Based on Plasma miRNAs Diagnoses Intrahepatic Cholangiocarcinoma: A Single Center with 1001 Samples
- PMID: 33805513
- PMCID: PMC8066692
- DOI: 10.3390/diagnostics11040610
A High-Accuracy Model Based on Plasma miRNAs Diagnoses Intrahepatic Cholangiocarcinoma: A Single Center with 1001 Samples
Abstract
Objectives: Intrahepatic cholangiocarcinoma (iCCA) is a highly malignant cancer. More than 70% of patients are diagnosed at an advanced stage. The aim of this study was to evaluate the diagnostic value of plasma miR-21, miR-122, and CA19-9, hoping to establish a novel model to improve the accuracy for diagnosing iCCA.
Materials and methods: Plasma miR-21 and miR-122 were detected in 359 iCCA patients and 642 controls (healthy, benign liver lesions, other malignant liver tumors). All 1001 samples were allocated to training cohort (n = 668) and validation cohort (n = 333) in a chronological order. A logistic regression model was applied to combine these markers. Area under the receiver operating characteristic curve (AUC) was used as an accuracy index to evaluate the diagnostic performance.
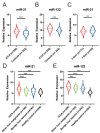
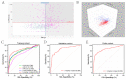
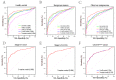
Results: Plasma miR-21 and miR-122 were significantly higher in iCCA patients than those in controls. Higher plasma miR-21 level was significantly correlated with larger tumor size (p = 0.030). A three-marker model was constructed by using miR-21, miR-122 and CA19-9, which showed an AUC of 0.853 (95% CI: 0.824-0.879; sensitivity: 73.0%, specificity: 87.4%) to differentiate iCCA from controls. These results were subsequently confirmed in the validation cohort with an AUC of 0.866 (0.825-0.901). The results were similar for diagnosing early (stages 0-I) iCCA patients (AUC: 0.848) and CA19-9negative iCCA patients (AUC: 0.795).
Conclusions: We established a novel three-marker model with a high accuracy based on a large number of participants to differentiate iCCA from controls. This model showed a great clinical value especially for the diagnosis of early iCCA and CA19-9negative iCCA.
Keywords: CA19-9; circulating biomarker; diagnosis; intrahepatic cholangiocarcinoma; microRNA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Banales J.M., Cardinale V., Carpino G., Marzioni M., Andersen J.B., Invernizzi P., Lind G.E., Folseraas T., Forbes S.J., Fouassier L., et al. Expert consensus document: Cholangiocarcinoma: Current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA) Nat. Rev. Gastroenterol. Hepatol. 2016;13:261–280. doi: 10.1038/nrgastro.2016.51. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

