Bacteriophages of Shiga Toxin-Producing Escherichia coli and Their Contribution to Pathogenicity
- PMID: 33805526
- PMCID: PMC8065619
- DOI: 10.3390/pathogens10040404
Bacteriophages of Shiga Toxin-Producing Escherichia coli and Their Contribution to Pathogenicity
Abstract
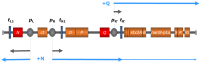
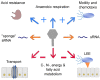
Shiga toxins (Stx) of Shiga toxin-producing Escherichia coli (STEC) are generally encoded in the genome of lambdoid bacteriophages, which spend the most time of their life cycle integrated as prophages in specific sites of the bacterial chromosome. Upon spontaneous induction or induction by chemical or physical stimuli, the stx genes are co-transcribed together with the late phase genes of the prophages. After being assembled in the cytoplasm, and after host cell lysis, mature bacteriophage particles are released into the environment, together with Stx. As members of the group of lambdoid phages, Stx phages share many genetic features with the archetypical temperate phage Lambda, but are heterogeneous in their DNA sequences due to frequent recombination events. In addition to Stx phages, the genome of pathogenic STEC bacteria may contain numerous prophages, which are either cryptic or functional. These prophages may carry foreign genes, some of them related to virulence, besides those necessary for the phage life cycle. Since the production of one or more Stx is considered the major pathogenicity factor of STEC, we aim to highlight the new insights on the contribution of Stx phages and other STEC phages to pathogenicity.
Keywords: STEC; Shiga toxins; Stx phages; antibiotic resistance genes; lambdoid prophages; pathogenicity; virulence factors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Datz M., Janetzki-Mittmann C., Franke S., Gunzer F., Schmidt H., Karch H. Analysis of the enterohemorrhagic Escherichia coli O157 DNA region containing lambdoid phage gene p and shiga-like toxin structural genes. Appl. Environ. Microbiol. 1996;62:791–797. doi: 10.1128/AEM.62.3.791-797.1996. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

