Dietary Marginal and Excess Selenium Increased Triglycerides Deposition, Induced Endoplasmic Reticulum Stress and Differentially Influenced Selenoproteins Expression in the Anterior and Middle Intestines of Yellow Catfish Pelteobagrus fulvidraco
- PMID: 33805536
- PMCID: PMC8067157
- DOI: 10.3390/antiox10040535
Dietary Marginal and Excess Selenium Increased Triglycerides Deposition, Induced Endoplasmic Reticulum Stress and Differentially Influenced Selenoproteins Expression in the Anterior and Middle Intestines of Yellow Catfish Pelteobagrus fulvidraco
Abstract
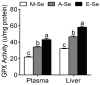
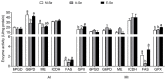
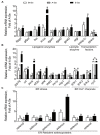
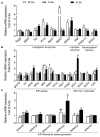
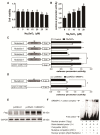
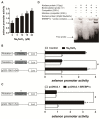
Selenium (Se) is an essential micro-mineral and plays important roles in antioxidant responses, and also influences lipid metabolism and selenoprotein expression in vertebrates, but the effects and mechanism remain unknown. The study was undertaken to decipher the insights into dietary Se influencing lipid metabolism and selenoprotein expression in the anterior and middle intestine (AI and MI) of yellow catfish Pelteobagrus fulvidraco. Yellow catfish (weight: 8.27 ± 0.03 g) were fed a 0.03- (M-Se), 0.25- (A-Se), or 6.39- (E-Se) mg Se/kg diet for 12 wk. AI and MI were analyzed for triglycerides (TGs) and Se concentrations, histochemistry and immunofluorescence, enzyme activities, and gene and protein levelsassociated with antioxidant responses, lipid metabolism, endoplasmic reticulum (ER) stress, and selenoproteome. Compared to the A-Se group, M-Se and E-Se diets significantly decreased weight gain (WG) and increased TGs concentration in the AI and MI. In the AI, compared with A-Se group, M-Se and E-Se diets significantly increased activities of fatty acid synthase, expression of lipogenic genes, and suppressed lipolysis. In the MI, compared to the A-Se group, M-Se and E-Se diets significantly increased activities of lipogenesis and expression of lipogenic genes. Compared with A-Se group, E-Se diet significantly increased glutathione peroxidase (GPX) activities in the AI and MI, and M-Se diet did not significantly reduce GPX activities in the AI and MI. Compared with the A- Se group, E-Se diet significantly increased glutathione peroxidase (GPX) activities in the plasma and liver, and M-Se diet significantly reduced GPX activities in the plasma and liver. Compared with the A-Se group, M-Se and E-Se groups also increased glucose-regulated protein 78 (GRP78, ER stress marker) protein expression of the intestine. Dietary Se supplementation also differentially influenced the expression of the 28 selenoproteins in the AI and MI, many of which possessed antioxidant characteristics. Compared with the A-Se group, the M-Se group significantly decreased mRNA levels of txnrd2 and txnrd3, but made no difference on mRNA levels of these seven GPX proteins in the MI. Moreover, we characterized sterol regulatory element binding protein 1c (SREBP1c) binding sites of three ER-resident proteins (selenom, selenon, and selenos) promoters, and found that Se positively controlled selenom, selenon, and selenos expression via SREBP1c binding to the selenom, selenon, and selenos promoter. Thus, dietary marginal and excess Se increased TGs deposition of yellow catfish P. fulvidraco, which might be mediated by ER-resident selenoproteins expression and ER stress.
Keywords: endoplasmic reticulum stress; lipid metabolism; selenium; selenotranscriptome; transcription regulation; vertebrates.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

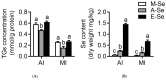


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

