Effects of Desiccation on Metamorphic Climax in Bombina variegata: Changes in Levels and Patterns of Oxidative Stress Parameters
- PMID: 33805554
- PMCID: PMC8066544
- DOI: 10.3390/ani11040953
Effects of Desiccation on Metamorphic Climax in Bombina variegata: Changes in Levels and Patterns of Oxidative Stress Parameters
Abstract
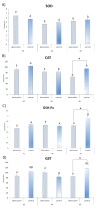
In this paper, we examined how the oxidative status (antioxidant system and oxidative damage) of Bombina variegata larvae changed during the metamorphic climax (Gosner stages: 42-beginning, 44-middle and 46-end) and compared the patterns and levels of oxidative stress parameters between individuals developing under constant water availability (control) and those developing under decreasing water availability (desiccation group). Our results revealed that larvae developing under decreasing water availability exhibited increased oxidative damage in the middle and end stages. This was followed by lower levels of glutathione in stages 44 and 46, as well as lower values of catalase, glutathione peroxidase, glutathione S-transferase and sulfhydryl groups in stage 46 (all in relation to control animals). Comparison between stages 42, 44 and 46 within treatments showed that individuals in the last stage demonstrated the highest intensities of lipid oxidative damage in both the control and desiccation groups. As for the parameters of the antioxidant system, control individuals displayed greater variety in response to changes induced by metamorphic climax than individuals exposed to desiccation treatment. The overall decrease in water availability during development led to increased oxidative stress and modifications in the pattern of AOS response to changes induced by metamorphic climax in larvae of B. variegata.
Keywords: amphibian larvae; antioxidant system; metamorphosis; oxidative damage; pond drying; yellow-bellied toad.
Conflict of interest statement
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. The contents of the manuscript have not been published previously, nor have they been submitted elsewhere for consideration, nor are they in press. All of the authors have seen and approved the manuscript. There are no competing interests and no conflicting financial, personal or other relationships with other persons or organizations.
Figures


Similar articles
-
Carry-Over Effects of Desiccation Stress on the Oxidative Status of Fasting Anuran Juveniles.Front Physiol. 2021 Dec 2;12:783288. doi: 10.3389/fphys.2021.783288. eCollection 2021. Front Physiol. 2021. PMID: 34925072 Free PMC article.
-
The role of phenotypic plasticity and corticosterone in coping with pond drying conditions in yellow-bellied toad (Bombina variegata, Linnaeus 1758) tadpoles.J Exp Zool A Ecol Integr Physiol. 2024 Aug;341(7):753-765. doi: 10.1002/jez.2819. Epub 2024 Apr 23. J Exp Zool A Ecol Integr Physiol. 2024. PMID: 38651613
-
Impact of desiccation pre-exposure on deltamethrin-induced oxidative stress in Bombina variegata juveniles.Comp Biochem Physiol C Toxicol Pharmacol. 2021 Dec;250:109191. doi: 10.1016/j.cbpc.2021.109191. Epub 2021 Sep 15. Comp Biochem Physiol C Toxicol Pharmacol. 2021. PMID: 34536572
-
Chronic effects of mercury on Bufo gargarizans larvae: Thyroid disruption, liver damage, oxidative stress and lipid metabolism disorder.Ecotoxicol Environ Saf. 2018 Nov 30;164:500-509. doi: 10.1016/j.ecoenv.2018.08.058. Epub 2018 Aug 23. Ecotoxicol Environ Saf. 2018. PMID: 30145490
-
Energetics of metamorphic climax in the southern toad (Bufo terrestris).Oecologia. 2003 Nov;137(3):344-51. doi: 10.1007/s00442-003-1374-5. Epub 2003 Sep 4. Oecologia. 2003. PMID: 12955492
Cited by
-
Protective Effects of α-Lipoic Acid and Chlorogenic Acid on Cadmium-Induced Liver Injury in Three-Yellow Chickens.Animals (Basel). 2021 May 29;11(6):1606. doi: 10.3390/ani11061606. Animals (Basel). 2021. PMID: 34072384 Free PMC article.
-
Antioxidant Response during the Kinetics of Anhydrobiosis in Two Eutardigrade Species.Life (Basel). 2022 May 30;12(6):817. doi: 10.3390/life12060817. Life (Basel). 2022. PMID: 35743848 Free PMC article.
-
Carry-Over Effects of Desiccation Stress on the Oxidative Status of Fasting Anuran Juveniles.Front Physiol. 2021 Dec 2;12:783288. doi: 10.3389/fphys.2021.783288. eCollection 2021. Front Physiol. 2021. PMID: 34925072 Free PMC article.
-
Oxidative Stress Parameters in Goitrogen-Exposed Crested Newt Larvae (Triturus spp.): Arrested Metamorphosis.Int J Environ Res Public Health. 2021 Sep 13;18(18):9653. doi: 10.3390/ijerph18189653. Int J Environ Res Public Health. 2021. PMID: 34574576 Free PMC article.
-
Natural modulation of redox status throughout the ontogeny of Amazon frog Physalaemus ephippifer (Anura, Leptodactylidae).Sci Rep. 2024 Sep 4;14(1):20655. doi: 10.1038/s41598-024-71022-0. Sci Rep. 2024. PMID: 39232193 Free PMC article.
References
-
- Sparling D.W., Linder G., Bishop C.A., Krest S. Ecotoxicology of Amphibians and Reptiles. CRC Press; Boca Raton, FL, USA: 2010.
-
- Gavrilović B.R., Petrović T.G., Radovanović T.B., Despotović S.G., Gavrić J.P., Krizmanić I.I., Ćirić M.D., Prokić M.D. Hepatic oxidative stress and neurotoxicity in Pelophylax kl. esculentus frogs: Influence of long-term exposure to a cyanobacterial bloom. Sci. Total Environ. 2021;750:141569. doi: 10.1016/j.scitotenv.2020.141569. - DOI - PubMed
-
- Blaustein A.R., Walls S.C., Bancroft B.A., Lawler J.J., Searle C.L., Gervasi S.S. Direct and indirect effects of climate change on amphibian populations. Diversity. 2010;2:281–313. doi: 10.3390/d2020281. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

