Physicochemical Properties of Lipoproteins Assessed by Nuclear Magnetic Resonance as a Predictor of Premature Cardiovascular Disease. PRESARV-SEA Study
- PMID: 33805580
- PMCID: PMC8037702
- DOI: 10.3390/jcm10071379
Physicochemical Properties of Lipoproteins Assessed by Nuclear Magnetic Resonance as a Predictor of Premature Cardiovascular Disease. PRESARV-SEA Study
Abstract
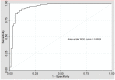
Some lipoprotein disorders related to the residual risk of premature cardiovascular disease (PCVD) are not detected by the conventional lipid profile. In this case-control study, the predictive power of PCVD of serum sdLDL-C, measured using a lipoprotein precipitation method, and of the physicochemical properties of serum lipoproteins, analyzed by nuclear magnetic resonance (NMR) techniques, were evaluated. We studied a group of patients with a first PCVD event (n = 125) and a group of control subjects (n = 190). Conventional lipid profile, the size and number of Very Low Density Lipoproteins (VLDL), Low Density Lipoproteins (LDL), High Density Lipoproteins (HDL) particles, and the number of particles of their subclasses (large, medium, and small) were measured. Compared to controls, PCVD patients had lower concentrations of all LDL particles, and smaller and larger diameter of LDL and HDL particles, respectively. PCVD patients also showed higher concentrations of small dense LDL-cholesterol (sdLDL), and triglycerides (Tg) in LDL and HDL particles (HDL-Tg), and higher concentrations of large VLDL particles. Multivariate logistic regression showed that sdLDL-C, HDL-Tg, and large concentrations of LDL particles were the most powerful predictors of PCVD. A strong relationship was observed between increased HDL-Tg concentrations and PCVD. This study demonstrates that beyond the conventional lipid profile, PCVD patients have other atherogenic lipoprotein alterations that are detected by magnetic resonance imaging (MRI) analysis.
Keywords: NMR analysis of lipoproteins; lipid profile; lipoprotein particle number; lipoprotein precipitation; premature cardiovascular disease; residual cardiovascular risk; small dense LDL.
Conflict of interest statement
Xavier Pintó declares personal fees from Amgen, Astra-Zeneca, Esteve, Ferrer, Merck, Mylan, Sanofi, outside the submitted work. Carlos Guijarro declares personal fees from AMGEN, Sanofi, Daiichi Sankyo, Ferrer Pfizer, Rubió, and MSD, related to lectures and advisory committees. Núria Amigó is stock owner of Biosfer Teslab and has a patent of the lipoprotein profiling described in the present manuscript. Pedro Valdivielso: advisory and lecture from Ferrer, Rubió, Amgen, Sanofi, MSD, Novartis, Amarin, Mylan, Daiichi-Sankyo, outside the submitted work. The other authors have no conflicts of interest to declare.
Figures
References
-
- Vikulova D.N., Grubisic M., Zhao Y., Lynch K., Humphries K.H., Pimstone S.N., Brunham L.R. Premature Atherosclerotic Cardiovascular Disease: Trends in Incidence, Risk Factors, and Sex-Related Differences, 2000 to 2016. J. Am. Heart Assoc. 2019;8:e012178. doi: 10.1161/JAHA.119.012178. - DOI - PMC - PubMed
-
- Piepoli M.F., Hoes A.W., Agewall S., Albus C., Brotons C., Catapano A.L., Cooney M.T., Corrà U., Cosyns B., Deaton C., et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR) Eur. Heart J. 2016;37:2315–2381. - PMC - PubMed
-
- Ambegaonkar B., Bash L., Chirovsky D., Jameson K., Grant S., Nocea G., Pettersson B., Sazonov V. Attainment of normal lipid levels among high cardiovascular risk patients: Pooles analysis of observational studies from the United Kingdom, Sweden, Spain and Canada. Eur. J. Intern. Med. 2013;24:656–663. doi: 10.1016/j.ejim.2013.07.005. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous