Generating a Precision Endoxifen Prediction Algorithm to Advance Personalized Tamoxifen Treatment in Patients with Breast Cancer
- PMID: 33805613
- PMCID: PMC8000933
- DOI: 10.3390/jpm11030201
Generating a Precision Endoxifen Prediction Algorithm to Advance Personalized Tamoxifen Treatment in Patients with Breast Cancer
Abstract
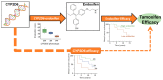
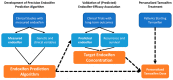
Tamoxifen is an endocrine treatment for hormone receptor positive breast cancer. The effectiveness of tamoxifen may be compromised in patients with metabolic resistance, who have insufficient metabolic generation of the active metabolites endoxifen and 4-hydroxy-tamoxifen. This has been challenging to validate due to the lack of measured metabolite concentrations in tamoxifen clinical trials. CYP2D6 activity is the primary determinant of endoxifen concentration. Inconclusive results from studies investigating whether CYP2D6 genotype is associated with tamoxifen efficacy may be due to the imprecision in using CYP2D6 genotype as a surrogate of endoxifen concentration without incorporating the influence of other genetic and clinical variables. This review summarizes the evidence that active metabolite concentrations determine tamoxifen efficacy. We then introduce a novel approach to validate this relationship by generating a precision endoxifen prediction algorithm and comprehensively review the factors that must be incorporated into the algorithm, including genetics of CYP2D6 and other pharmacogenes. A precision endoxifen algorithm could be used to validate metabolic resistance in existing tamoxifen clinical trial cohorts and could then be used to select personalized tamoxifen doses to ensure all patients achieve adequate endoxifen concentrations and maximum benefit from tamoxifen treatment.
Keywords: 4OHtam; CYP2C; CYP2D6; CYP3A; SULT; UGT; endoxifen; personalized treatment; pharmacogenetics; tamoxifen metabolism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Stearns V., Johnson M.D., Rae J.M., Morocho A., Novielli A., Bhargava P., Hayes D.F., Desta Z., Flockhart D.A. Active Tamoxifen Metabolite Plasma Concentrations After Coadministration of Tamoxifen and the Selective Serotonin Reuptake Inhibitor Paroxetine. J. Natl. Cancer Inst. 2003;95:1758–1764. doi: 10.1093/jnci/djg108. - DOI - PubMed
-
- Madlensky L., Natarajan L., Tchu S., Pu M., Mortimer J., Flatt S.W., Nikoloff D.M., Hillman G., Fontecha M.R., Lawrence H.J., et al. Tamoxifen Metabolite Concentrations, CYP2D6 Genotype, and Breast Cancer Outcomes. Clin. Pharmacol. Ther. 2011;89:718–725. doi: 10.1038/clpt.2011.32. - DOI - PMC - PubMed
-
- Saladores P., Murdter T., Eccles D., Chowbay B., Zgheib N.K., Winter S., Ganchev B., Eccles B., Gerty S., Tfayli A., et al. Tamoxifen metabolism predicts drug concentrations and outcome in premenopausal patients with early breast cancer. Pharm. J. 2015;15:84–94. doi: 10.1038/tpj.2014.34. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

