Saccharomyces cerevisiae Cell Wall-Based Adsorbent Reduces Aflatoxin B1 Absorption in Rats
- PMID: 33805637
- PMCID: PMC7999883
- DOI: 10.3390/toxins13030209
Saccharomyces cerevisiae Cell Wall-Based Adsorbent Reduces Aflatoxin B1 Absorption in Rats
Abstract
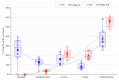
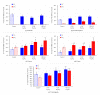
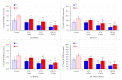
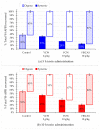
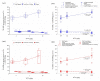
Mycotoxins are naturally occurring toxins that can affect livestock health and performance upon consumption of contaminated feedstuffs. To mitigate the negative effects of mycotoxins, sequestering agents, adsorbents, or binders can be included to feed to interact with toxins, aiding their passage through the gastrointestinal tract (GI) and reducing their bioavailability. The parietal cell wall components of Saccharomyces cerevisiae have been found to interact in vitro with mycotoxins, such as, but not limited to, aflatoxin B1 (AFB1), and to improve animal performance when added to contaminated diets in vivo. The present study aimed to examine the pharmacokinetics of the absorption of radiolabeled AFB1 in rats in the presence of a yeast cell wall-based adsorbent (YCW) compared with that in the presence of the clay-based binder hydrated sodium calcium aluminosilicate (HSCAS). The results of the initial pharmacokinetic analysis showed that the absorption process across the GI tract was relatively slow, occurring over a matter of hours rather than minutes. The inclusion of mycotoxin binders increased the recovery of radiolabeled AFB1 in the small intestine, cecum, and colon at 5 and 10 h, revealing that they prevented AFB1 absorption compared with a control diet. Additionally, the accumulation of radiolabeled AFB1 was more significant in the blood plasma, kidney, and liver of animals fed the control diet, again showing the ability of the binders to reduce the assimilation of AFB1 into the body. The results showed the potential of YCW in reducing the absorption of AFB1 in vivo, and in protecting against the damaging effects of AFB1 contamination.
Keywords: absorption; adsorption; aflatoxin B1; animal health; binder; mycotoxins; yeast cell wall.
Conflict of interest statement
The authors A.Y., G.P.D. and C.A.M. are employees of Alltech which produces and markets Mycosorb®, the commercial adsorbent evaluated in this study.
Figures


References
-
- Yiannikouris A., Jouany J.-P. Mycotoxins in feeds and their fate in animals: A review. Anim. Res. 2002;51:81–99. doi: 10.1051/animres:2002012. - DOI
-
- CAST . Mycotoxins: Risks in Plant, Animal, and Human Systems. Council for Agricultural Science and Technology; Ames, IA, USA: 2003.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

