Design, Synthesis, Molecular Modeling and Antitumor Evaluation of Novel Indolyl-Pyrimidine Derivatives with EGFR Inhibitory Activity
- PMID: 33805918
- PMCID: PMC8037142
- DOI: 10.3390/molecules26071838
Design, Synthesis, Molecular Modeling and Antitumor Evaluation of Novel Indolyl-Pyrimidine Derivatives with EGFR Inhibitory Activity
Abstract
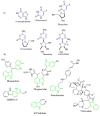
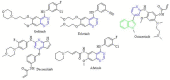
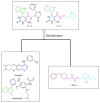
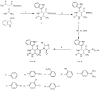
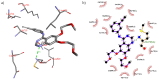
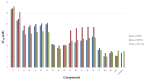
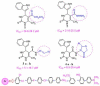
Scaffolds hybridization is a well-known drug design strategy for antitumor agents. Herein, series of novel indolyl-pyrimidine hybrids were synthesized and evaluated in vitro and in vivo for their antitumor activity. The in vitro antiproliferative activity of all compounds was obtained against MCF-7, HepG2, and HCT-116 cancer cell lines, as well as against WI38 normal cells using the resazurin assay. Compounds 1-4 showed broad spectrum cytotoxic activity against all these cancer cell lines compared to normal cells. Compound 4g showed potent antiproliferative activity against these cell lines (IC50 = 5.1, 5.02, and 6.6 μM, respectively) comparable to the standard treatment (5-FU and erlotinib). In addition, the most promising group of compounds was further evaluated for their in vivo antitumor efficacy against EAC tumor bearing mice. Notably, compound 4g showed the most potent in vivo antitumor activity. The most active compounds were evaluated for their EGFR inhibitory (range 53-79%) activity. Compound 4g was found to be the most active compound against EGFR (IC50 = 0.25 µM) showing equipotency as the reference treatment (erlotinib). Molecular modeling study was performed on compound 4g revealed a proper binding of this compound inside the EGFR active site comparable to erlotinib. The data suggest that compound 4g could be used as a potential anticancer agent.
Keywords: EGFR; cancer; drug design; indole; molecular modeling; pyrimidine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

