Skin Wound-Healing Potential of Polysaccharides from Medicinal Mushroom Auricularia auricula-judae (Bull.)
- PMID: 33806146
- PMCID: PMC8064461
- DOI: 10.3390/jof7040247
Skin Wound-Healing Potential of Polysaccharides from Medicinal Mushroom Auricularia auricula-judae (Bull.)
Abstract
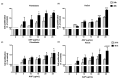
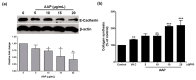
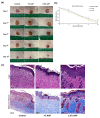
Auricularia auricula-judae, a nutrient-rich mushroom used in traditional medicine, is a macrofungi that exhibits various biological properties. In this study, we have reported on the mechanisms that promote the wound-healing effects of a water-soluble polysaccharide-rich extract obtained from A. auricula-judae (AAP). AAP contained high amounts of polysaccharides (349.83 ± 5.00 mg/g extract) with a molecular weight of 158 kDa. The main sugar composition of AAP includes mannose, galactose, and glucose. AAP displayed antioxidant activity in vitro and was able to abort UVB-induced intracellular ROS production in human fibroblasts in cellulo. AAP significantly promoted both fibroblast and keratinocyte proliferation, migration, and invasion, along with augmentation of the wound-healing process by increasing collagen synthesis and decreasing E-cadherin expression (All p < 0.05). Specifically, the AAP significantly accelerated the wound closure in a mice skin wound-healing model on day 9 (2.5%AAP, p = 0.031 vs. control) and day 12 (1% and 2.5%AAP with p = 0.009 and p < 0.001 vs. control, respectively). Overall, our results indicate that the wound-healing activities of AAP can be applied in an AAP-based product for wound management.
Keywords: Auricularia auricula-judae; collagen synthesis; fibroblasts proliferation; in vivo skin wound healing; keratinocytes proliferation; medicinal mushroom; polysaccharide-rich extract; wound healing.
Conflict of interest statement
The authors declare no conflict of interest and the funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

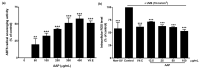

References
-
- Enoch S., Leaper D.J. Basic science of wound healing. Surgery. 2008;26:31–37.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

