Fluorine-19 Magnetic Resonance Imaging for Detection of Amyloid β Oligomers Using a Keto Form of Curcumin Derivative in a Mouse Model of Alzheimer's Disease
- PMID: 33806326
- PMCID: PMC7961357
- DOI: 10.3390/molecules26051362
Fluorine-19 Magnetic Resonance Imaging for Detection of Amyloid β Oligomers Using a Keto Form of Curcumin Derivative in a Mouse Model of Alzheimer's Disease
Abstract
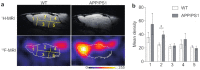
Recent evidence suggests that the formation of soluble amyloid β (Aβ) aggregates with high toxicity, such as oligomers and protofibrils, is a key event that causes Alzheimer's disease (AD). However, understanding the pathophysiological role of such soluble Aβ aggregates in the brain in vivo could be difficult due to the lack of a clinically available method to detect, visualize, and quantify soluble Aβ aggregates in the brain. We had synthesized a novel fluorinated curcumin derivative with a fixed keto form, named as Shiga-Y51, which exhibited high selectivity to Aβ oligomers in vitro. In this study, we investigated the in vivo detection of Aβ oligomers by fluorine-19 (19F) magnetic resonance imaging (MRI) using Shiga-Y51 in an APP/PS1 double transgenic mouse model of AD. Significantly high levels of 19F signals were detected in the upper forebrain region of APP/PS1 mice compared with wild-type mice. Moreover, the highest levels of Aβ oligomers were detected in the upper forebrain region of APP/PS1 mice in enzyme-linked immunosorbent assay. These findings suggested that 19F-MRI using Shiga-Y51 detected Aβ oligomers in the in vivo brain. Therefore, 19F-MRI using Shiga-Y51 with a 7 T MR scanner could be a powerful tool for imaging Aβ oligomers in the brain.
Keywords: Alzheimer’s disease; curcumin; imaging biomarker; keto-enol tautomerism; magnetic resonance imaging; mouse model.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Hardy J.A., Higgins G.A. Higgins Alzheimer’s Disease: The Amyloid Cascade Hypothesis. Science. 1992;256:184–185. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

