Genetic Regulation of Physiological Reproductive Lifespan and Female Fertility
- PMID: 33806348
- PMCID: PMC7961500
- DOI: 10.3390/ijms22052556
Genetic Regulation of Physiological Reproductive Lifespan and Female Fertility
Abstract
There is substantial genetic variation for common traits associated with reproductive lifespan and for common diseases influencing female fertility. Progress in high-throughput sequencing and genome-wide association studies (GWAS) have transformed our understanding of common genetic risk factors for complex traits and diseases influencing reproductive lifespan and fertility. The data emerging from GWAS demonstrate the utility of genetics to explain epidemiological observations, revealing shared biological pathways linking puberty timing, fertility, reproductive ageing and health outcomes. The observations also identify unique genetic risk factors specific to different reproductive diseases impacting on female fertility. Sequencing in patients with primary ovarian insufficiency (POI) have identified mutations in a large number of genes while GWAS have revealed shared genetic risk factors for POI and ovarian ageing. Studies on age at menopause implicate DNA damage/repair genes with implications for follicle health and ageing. In addition to the discovery of individual genes and pathways, the increasingly powerful studies on common genetic risk factors help interpret the underlying relationships and direction of causation in the regulation of reproductive lifespan, fertility and related traits.
Keywords: AMH; FSH; Keywords: reproductive lifespan; fertility; genetic variation; menopause; review.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
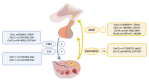
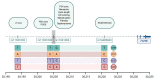

References
-
- Buniello A., MacArthur J.A.L., Cerezo M., Harris L.W., Hayhurst J., Malangone C., McMahon A., Morales J., Mountjoy E., Sollis E., et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 2019;47:D1005–D1012. doi: 10.1093/nar/gky1120. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

