A Commonly Used Biocide 2-N-octyl-4-isothiazolin-3-oneInduces Blood-Brain Barrier Dysfunction via Cellular Thiol Modification and Mitochondrial Damage
- PMID: 33806369
- PMCID: PMC7975974
- DOI: 10.3390/ijms22052563
A Commonly Used Biocide 2-N-octyl-4-isothiazolin-3-oneInduces Blood-Brain Barrier Dysfunction via Cellular Thiol Modification and Mitochondrial Damage
Abstract
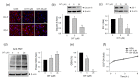
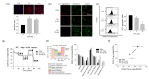
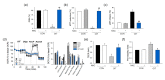
Isothiazolinone (IT) biocides are potent antibacterial substances commonly used as preservatives or disinfectants, and 2-n-Octyl-4-isothiazolin-3-one (OIT; octhilinone) is a common IT biocide that is present in leather products, glue, paints, and cleaning products. Although humans are exposed to OIT through personal and industrial use, the potentially deleterious effects of OIT on human health are still unknown. To investigate the effects of OIT on the vascular system, which is continuously exposed to xenobiotics through systemic circulation, we treated brain endothelial cells with OIT. OIT treatment significantly activated caspase-3-mediated apoptosis and reduced the bioenergetic function of mitochondria in a bEnd.3 cell-based in vitro blood-brain barrier (BBB) model. Interestingly, OIT significantly altered the thiol redox status, as evidenced by reduced glutathione levels and protein S-nitrosylation. The endothelial barrier function of bEnd.3 cells was significantly impaired by OIT treatment. OIT affected mitochondrial dynamics through mitophagy and altered mitochondrial morphology in bEnd.3 cells. N-acetyl cysteine significantly reversed the effects of OIT on the metabolic capacity and endothelial function of bEnd.3 cells. Taken together, we demonstrated that the alteration of the thiol redox status and mitochondrial damage contributed to OIT-induced BBB dysfunction, and we hope that our findings will improve our understanding of the potential hazardous health effects of IT biocides.
Keywords: 2-n-Octyl-4-isothiazolin-3-one (OIT); blood–brain barrier (BBB) model; isothiazolinone (IT) biocide; mitochondrial dysfunction; oxidative stress; protein S-nitrosylation (SNO).
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Comparative study of two isothiazolinone biocides, 1,2-benzisothiazolin-3-one (BIT) and 4,5-dichloro-2-n-octyl-isothiazolin-3-one (DCOIT), on barrier function and mitochondrial bioenergetics using murine brain endothelial cell line (bEND.3).J Toxicol Environ Health A. 2021 Nov 17;84(22):932-943. doi: 10.1080/15287394.2021.1955786. Epub 2021 Jul 27. J Toxicol Environ Health A. 2021. PMID: 34315345
-
ATN-161 Ameliorates Ischemia/Reperfusion-induced Oxidative Stress, Fibro-inflammation, Mitochondrial damage, and Apoptosis-mediated Tight Junction Disruption in bEnd.3 Cells.Inflammation. 2021 Dec;44(6):2377-2394. doi: 10.1007/s10753-021-01509-9. Epub 2021 Aug 22. Inflammation. 2021. PMID: 34420157 Free PMC article.
-
Functional and dynamic mitochondrial damage by chloromethylisothiazolinone/methylisothiazolinone (CMIT/MIT) mixture in brain endothelial cell lines and rat cerebrovascular endothelium.Toxicol Lett. 2022 Aug 1;366:45-57. doi: 10.1016/j.toxlet.2022.06.010. Epub 2022 Jul 5. Toxicol Lett. 2022. PMID: 35803525
-
Mitochondrial Changes in Rat Brain Endothelial Cells Associated with Hepatic Encephalopathy: Relation to the Blood-Brain Barrier Dysfunction.Neurochem Res. 2024 Jun;49(6):1489-1504. doi: 10.1007/s11064-022-03698-7. Epub 2022 Aug 2. Neurochem Res. 2024. PMID: 35917006 Free PMC article. Review.
-
Involvement of ROS in BBB dysfunction.Free Radic Res. 2009 Apr;43(4):348-64. doi: 10.1080/10715760902751902. Epub 2009 Feb 24. Free Radic Res. 2009. PMID: 19241241 Review.
Cited by
-
Conventional and innovative approaches to black fungi control for stone heritage preservation.IUBMB Life. 2025 Mar;77(3):e70010. doi: 10.1002/iub.70010. IUBMB Life. 2025. PMID: 40111005 Free PMC article. Review.
-
2-(4-Nitrophenyl)isothiazol-3(2H)-one: A Promising Selective Agent against Hepatocellular Carcinoma Cells.Pharmaceuticals (Basel). 2024 May 24;17(6):673. doi: 10.3390/ph17060673. Pharmaceuticals (Basel). 2024. PMID: 38931341 Free PMC article.
-
Allergic contact dermatitis caused by octylisothiazolinone in a leather car seat: Case report and emergence of octylisothiazolinones in leather goods in Switzerland.Contact Dermatitis. 2022 Nov;87(5):455-457. doi: 10.1111/cod.14188. Epub 2022 Jul 22. Contact Dermatitis. 2022. PMID: 35834722 Free PMC article. No abstract available.
-
The Molecular Basis for the Environmental Promotion of Neurodegenerative Disease.Int J Mol Sci. 2023 Mar 25;24(7):6209. doi: 10.3390/ijms24076209. Int J Mol Sci. 2023. PMID: 37047180 Free PMC article.
-
Carnosine Protects against Cerebral Ischemic Injury by Inhibiting Matrix-Metalloproteinases.Int J Mol Sci. 2021 Jul 13;22(14):7495. doi: 10.3390/ijms22147495. Int J Mol Sci. 2021. PMID: 34299128 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

