Pyrvinium Treatment Confers Hepatic Metabolic Benefits via β-Catenin Downregulation and AMPK Activation
- PMID: 33806415
- PMCID: PMC8001320
- DOI: 10.3390/pharmaceutics13030330
Pyrvinium Treatment Confers Hepatic Metabolic Benefits via β-Catenin Downregulation and AMPK Activation
Retraction in
-
RETRACTED: Zhou et al. Pyrvinium Treatment Confers Hepatic Metabolic Benefits via β-Catenin Downregulation and AMPK Activation. Pharmaceutics 2021, 13, 330.Pharmaceutics. 2023 May 30;15(6):1623. doi: 10.3390/pharmaceutics15061623. Pharmaceutics. 2023. PMID: 37376227 Free PMC article.
Abstract
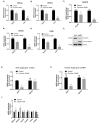
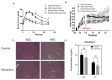
Genetic evidence has indicated that β-catenin plays a vital role in glucose and lipid metabolism. Here, we investigated whether pyrvinium, an anthelmintic agent previously reported as a down-regulator of cellular β-catenin levels, conferred any metabolic advantages in treatment of metabolic disorders. Glucose production and lipid accumulation were analyzed to assess metabolic response to pyrvinium in hepatocytes. The expression of key proteins and genes were assessed by immunoblotting and RT-PCR. The in vivo efficacy of pyrvinium against metabolic disorders was evaluated in the mice fed with a high fat diet (HFD). We found that pyrvinium inhibited glucose production and reduced lipogenesis by decreasing the expression of key genes in hepatocytes, which were partially elicited by the downregulation of β-catenin through AXIN stabilization. Interestingly, the AMPK pathway also played a role in the action of pyrvinium, dependent on AXIN stabilization but independent of β-catenin downregulation. In HFD-fed mice, pyrvinium treatment led to improvement in glucose tolerance, fatty liver disorder, and serum cholesterol levels along with a reduced body weight gain. Our results show that small molecule stabilization of AXIN using pyrvinium may lead to improved glucose and lipid metabolism, via β-catenin downregulation and AMPK activation.
Keywords: AMPK activation; beta catenin; gluconeogenesis; lipid metabolism; pyrvinium.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures





References
-
- Behari J., Li H., Liu S., Stefanovic-Racic M., Alonso L., O’Donnell C.P., Shiva S., Singamsetty S., Watanabe Y., Singh V.P., et al. beta-catenin links hepatic metabolic zonation with lipid metabolism and diet-induced obesity in mice. Am. J. Pathol. 2014;184:3284–3298. doi: 10.1016/j.ajpath.2014.08.022. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

