The Roadmap of Colorectal Cancer Screening
- PMID: 33806465
- PMCID: PMC7961708
- DOI: 10.3390/cancers13051101
The Roadmap of Colorectal Cancer Screening
Abstract
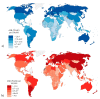
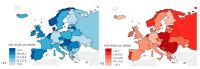
Colorectal cancer (CRC) is the third most common form of cancer in terms of incidence and the second in terms of mortality worldwide. CRC develops over several years, thus highlighting the importance of early diagnosis. National screening programs based on fecal occult blood tests and subsequent colonoscopy have reduced the incidence and mortality, however improvements are needed since the participation rate remains low and the tests present a high number of false positive results. This review provides an overview of the CRC screening globally and the state of the art in approaches aimed at improving accuracy and participation in CRC screening, also considering the need for gender and age differentiation. New fecal tests and biomarkers such as DNA methylation, mutation or integrity, proteins and microRNAs are explored, including recent investigations into fecal microbiota. Liquid biopsy approaches, involving novel biomarkers and panels, such as circulating mRNA, micro- and long-non-coding RNA, DNA, proteins and extracellular vesicles are discussed. The approaches reported are based on quantitative PCR methods that could be easily applied to routine screening, or arrays and sequencing assays that should be better exploited to describe and identify candidate biomarkers in blood samples.
Keywords: colonoscopy; ctDNA; extracellular vesicles; fecal immunochemical test (FIT); flexible sigmoidoscopy; liquid biopsy; mRNA; microRNA; proteins.
Conflict of interest statement
R.S. and M.L. have intellectual property rights on an international patent pending (WO2016/185451: method and kit for diagnosis of colorectal cancer; USA patent number 10900085; European patent office number EP3298165). R.S., L.R. and M.L. have intellectual property rights on an international patent pending (WO/2019/138303: new prognostic method). The remaining authors (E.F. and M.S.) declared no conflict of interest. The funders had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- Puccini A., Berger M.D., Naseem M., Tokunaga R., Battaglin F., Cao S., Hanna D.L., McSkane M., Soni S., Zhang W., et al. Colorectal cancer: Epigenetic alterations and their clinical implications. Biochim. Biophys. Acta Rev. Cancer. 2017;1868:439–448. doi: 10.1016/j.bbcan.2017.09.003. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

