Evaluation of the Interactions between Human Serum Albumin (HSA) and Non-Steroidal Anti-Inflammatory (NSAIDs) Drugs by Multiwavelength Molecular Fluorescence, Structural and Computational Analysis
- PMID: 33806467
- PMCID: PMC8000696
- DOI: 10.3390/ph14030214
Evaluation of the Interactions between Human Serum Albumin (HSA) and Non-Steroidal Anti-Inflammatory (NSAIDs) Drugs by Multiwavelength Molecular Fluorescence, Structural and Computational Analysis
Abstract
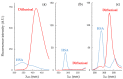
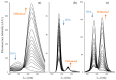
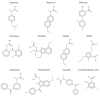
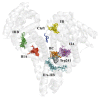
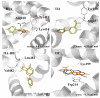
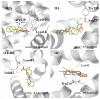
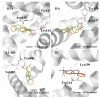
The interaction between drugs and transport proteins, such as albumins, is a key factor in drug bioavailability. One of the techniques commonly used for the evaluation of the drug-protein complex formation is fluorescence. This work studies the interaction of human serum albumin (HSA) with four non-steroidal anti-inflammatory drugs (NSAIDs)-ibuprofen, flurbiprofen, naproxen, and diflunisal-by monitoring the fluorescence quenching when the drug-albumin complex is formed. Two approaches-the double logarithm Stern-Volmer equation and the STAR program-are used to evaluate the binding parameters. The results are analyzed considering the binding properties, determined by using other complementary techniques and the available structural information of albumin complexes with NSAID-related compounds. Finally, this combined analysis has been synergistically used to interpret the binding of flurbiprofen to HSA.
Keywords: drug-protein interactions; fluorescence multiwavelength data treatment; fluorescence quenching; human serum albumin; molecular modeling; non-steroidal anti-inflammatory drugs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Molecular interactions between some non-steroidal anti-inflammatory drugs (NSAID's) and bovine (BSA) or human (HSA) serum albumin estimated by means of isothermal titration calorimetry (ITC) and frontal analysis capillary electrophoresis (FA/CE).Talanta. 2014 Dec;130:241-50. doi: 10.1016/j.talanta.2014.06.060. Epub 2014 Jul 5. Talanta. 2014. PMID: 25159405
-
A fluorescent spectroscopy and modelling analysis of anti-heparanase aptamers-serum protein interactions.J Photochem Photobiol B. 2013 Oct 5;127:68-77. doi: 10.1016/j.jphotobiol.2013.06.015. Epub 2013 Aug 3. J Photochem Photobiol B. 2013. PMID: 23968994
-
A Comprehensive Spectroscopic Analysis of the Ibuprofen Binding with Human Serum Albumin, Part I.Pharmaceuticals (Basel). 2020 Aug 21;13(9):205. doi: 10.3390/ph13090205. Pharmaceuticals (Basel). 2020. PMID: 32825638 Free PMC article.
-
Binding Studies of AICAR and Human Serum Albumin by Spectroscopic, Theoretical, and Computational Methodologies.Molecules. 2020 Nov 19;25(22):5410. doi: 10.3390/molecules25225410. Molecules. 2020. PMID: 33228044 Free PMC article.
-
Binding of naproxen enantiomers to human serum albumin studied by fluorescence and room-temperature phosphorescence.Spectrochim Acta A Mol Biomol Spectrosc. 2013 Mar 15;105:67-73. doi: 10.1016/j.saa.2012.12.007. Epub 2012 Dec 14. Spectrochim Acta A Mol Biomol Spectrosc. 2013. PMID: 23295212
Cited by
-
NMR Investigation of the Interaction of Three Non-Steroidal Anti-Inflammatory Drugs with Human Serum Albumin.Molecules. 2022 Oct 6;27(19):6647. doi: 10.3390/molecules27196647. Molecules. 2022. PMID: 36235184 Free PMC article.
-
Revisiting and Updating the Interaction between Human Serum Albumin and the Non-Steroidal Anti-Inflammatory Drugs Ketoprofen and Ketorolac.Molecules. 2024 Jun 24;29(13):3001. doi: 10.3390/molecules29133001. Molecules. 2024. PMID: 38998953 Free PMC article.
-
Interaction of the Emerging Mycotoxins Beauvericin, Cyclopiazonic Acid, and Sterigmatocystin with Human Serum Albumin.Biomolecules. 2022 Aug 11;12(8):1106. doi: 10.3390/biom12081106. Biomolecules. 2022. PMID: 36009000 Free PMC article.
-
Interaction of Ibuprofen with Partially Unfolded Bovine Serum Albumin in the Presence of Ionic Micelles and Oligosaccharides at Different λex and pH: A Spectroscopic Analysis.ACS Omega. 2023 Jan 10;8(3):3114-3128. doi: 10.1021/acsomega.2c06447. eCollection 2023 Jan 24. ACS Omega. 2023. PMID: 36713709 Free PMC article.
-
Albumin-Hyaluronan Interactions: Influence of Ionic Composition Probed by Molecular Dynamics.Int J Mol Sci. 2021 Nov 16;22(22):12360. doi: 10.3390/ijms222212360. Int J Mol Sci. 2021. PMID: 34830249 Free PMC article.
References
-
- FDA The Drug Development Process. [(accessed on 15 January 2021)]; Available online: https://www.fda.gov/ForPatients/Approvals/Drugs/default.htm.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

