Changes in Representation of Thalamic Projection Neurons within Prefrontal-Thalamic-Hippocampal Circuitry in a Rat Model of Third Trimester Binge Drinking
- PMID: 33806485
- PMCID: PMC8001051
- DOI: 10.3390/brainsci11030323
Changes in Representation of Thalamic Projection Neurons within Prefrontal-Thalamic-Hippocampal Circuitry in a Rat Model of Third Trimester Binge Drinking
Abstract
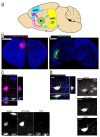
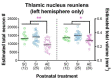
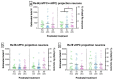
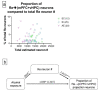
Alcohol exposure (AE) during the third trimester of pregnancy-a period known as the brain growth spurt (BGS)-could result in a diagnosis of a fetal alcohol spectrum disorder (FASD), a hallmark of which is impaired executive functioning (EF). Coordinated activity between prefrontal cortex and hippocampus is necessary for EF and thalamic nucleus reuniens (Re), which is required for prefrontal-hippocampal coordination, is damaged following high-dose AE during the BGS. The current experiment utilized high-dose AE (5.25 g/kg/day) during the BGS (i.e., postnatal days 4-9) of Long-Evans rat pups. AE reduces the number of neurons in Re into adulthood and selectively alters the proportion of Re neurons that simultaneously innervate both medial prefrontal cortex (mPFC) and ventral hippocampus (vHPC). The AE-induced change unique to Re→(mPFC + vHPC) projection neurons (neuron populations that innervate either mPFC or vHPC individually were unchanged) is not mediated by reduction in neuron number. These data are the first to examine mPFC-Re-HPC circuit connectivity in a rodent model of FASD, and suggest that both short-term AE-induced neuron loss and long-term changes in thalamic connectivity may be two distinct (but synergistic) mechanisms by which developmental AE can alter mPFC-Re-vHPC circuitry and impair EF throughout the lifespan.
Keywords: AAV; alcohol; connectivity; development; fetal alcohol spectrum disorders (FASD); immunofluorescence; immunohistochemistry; thalamus; unbiased stereology.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

