Small Immunomodulatory Molecules as Potential Therapeutics in Experimental Murine Models of Acute Lung Injury (ALI)/Acute Respiratory Distress Syndrome (ARDS)
- PMID: 33806560
- PMCID: PMC7961996
- DOI: 10.3390/ijms22052573
Small Immunomodulatory Molecules as Potential Therapeutics in Experimental Murine Models of Acute Lung Injury (ALI)/Acute Respiratory Distress Syndrome (ARDS)
Abstract
Background: Acute lung injury (ALI) or its most advanced form, acute respiratory distress syndrome (ARDS) is a severe inflammatory pulmonary process triggered by a variety of insults including sepsis, viral or bacterial pneumonia, and mechanical ventilator-induced trauma. Currently, there are no effective therapies available for ARDS. We have recently reported that a novel small molecule AVR-25 derived from chitin molecule (a long-chain polymer of N-acetylglucosamine) showed anti-inflammatory effects in the lungs. The goal of this study was to determine the efficacy of two chitin-derived compounds, AVR-25 and AVR-48, in multiple mouse models of ALI/ARDS. We further determined the safety and pharmacokinetic (PK) profile of the lead compound AVR-48 in rats.
Methods: ALI in mice was induced by intratracheal instillation of a single dose of lipopolysaccharide (LPS; 100 µg) for 24 h or exposed to hyperoxia (100% oxygen) for 48 h or undergoing cecal ligation and puncture (CLP) procedure and observation for 10 days.
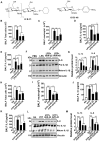
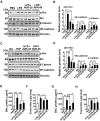
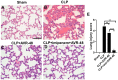
Results: Both chitin derivatives, AVR-25 and AVR-48, showed decreased neutrophil recruitment and reduced inflammation in the lungs of ALI mice. Further, AVR-25 and AVR-48 mediated diminished lung inflammation was associated with reduced expression of lung adhesion molecules with improvement in pulmonary endothelial barrier function, pulmonary edema, and lung injury. Consistent with these results, CLP-induced sepsis mice treated with AVR-48 showed a significant increase in survival of the mice (80%) and improved lung histopathology in the treated CLP group. AVR-48, the lead chitin derivative compound, demonstrated a good safety profile.
Conclusion: Both AVR-25 and AVR-48 demonstrate the potential to be developed as therapeutic agents to treat ALI/ARDS.
Keywords: AVR-25; AVR-48; acute lung injury; lung inflammation; pulmonary edema; sepsis.
Conflict of interest statement
The work done by D.S., P.D. and V.B. was funded partly by AyuVis Research, Inc., as a contract research award. B.A., and D.C. received compensation from AyuVis as consultants and S.M.R. received compensation from AyuVis as a member of the scientific advisory board and as consultant. S.A. received compensation as part time employee from AyuVis Research, Inc. The funders had a role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results, as described above.
Figures




References
-
- Mora-Rillo M., Arsuaga M., Ramirez-Olivencia G., de la Calle F., Borobia A.M., Sanchez-Seco P., Lago M., Figueira J.C., Fernandez-Puntero B., Viejo A., et al. Acute respiratory distress syndrome after convalescent plasma use: Treatment of a patient with Ebola virus disease contracted in Madrid, Spain. Lancet Respir. Med. 2015;3:554–562. doi: 10.1016/S2213-2600(15)00180-0. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

