Oxidized Phospholipids in Tumor Microenvironment Stimulate Tumor Metastasis via Regulation of Autophagy
- PMID: 33806593
- PMCID: PMC8001732
- DOI: 10.3390/cells10030558
Oxidized Phospholipids in Tumor Microenvironment Stimulate Tumor Metastasis via Regulation of Autophagy
Abstract
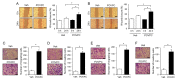
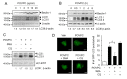
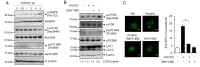
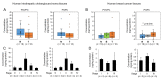
Oxidized phospholipids are well known to play physiological and pathological roles in regulating cellular homeostasis and disease progression. However, their role in cancer metastasis has not been entirely understood. In this study, effects of oxidized phosphatidylcholines such as 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphocholine (POVPC) on epithelial-mesenchymal transition (EMT) and autophagy were determined in cancer cells by immunoblotting and confocal analysis. Metastasis was analyzed by a scratch wound assay and a transwell migration/invasion assay. The concentrations of POVPC and 1-palmitoyl-2-glutaroyl-sn-glycero-phosphocholine (PGPC) in tumor tissues obtained from patients were measured by LC-MS/MS analysis. POVPC induced EMT, resulting in increase of migration and invasion of human hepatocellular carcinoma cells (HepG2) and human breast cancer cells (MCF7). POVPC induced autophagic flux through AMPK-mTOR pathway. Pharmacological inhibition or siRNA knockdown of autophagy decreased migration and invasion of POVPC-treated HepG2 and MCF7 cells. POVPC and PGPC levels were greatly increased at stage II of patient-derived intrahepatic cholangiocarcinoma tissues. PGPC levels were higher in malignant breast tumor tissues than in adjacent nontumor tissues. The results show that oxidized phosphatidylcholines increase metastatic potential of cancer cells by promoting EMT, mediated through autophagy. These suggest the positive regulatory role of oxidized phospholipids accumulated in tumor microenvironment in the regulation of tumorigenesis and metastasis.
Keywords: autophagy; cancer; metastasis; oxidative stress; phospholipids.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
The Levels of Oxidized Phospholipids in High-Density Lipoprotein During the Course of Sepsis and Their Prognostic Value.Front Immunol. 2022 May 3;13:893929. doi: 10.3389/fimmu.2022.893929. eCollection 2022. Front Immunol. 2022. PMID: 35592322 Free PMC article.
-
Toxicity of oxidized phospholipids in cultured macrophages.Lipids Health Dis. 2012 Sep 7;11:110. doi: 10.1186/1476-511X-11-110. Lipids Health Dis. 2012. PMID: 22958747 Free PMC article.
-
Structurally similar oxidized phospholipids differentially regulate endothelial binding of monocytes and neutrophils.Proc Natl Acad Sci U S A. 1999 Oct 12;96(21):12010-5. doi: 10.1073/pnas.96.21.12010. Proc Natl Acad Sci U S A. 1999. PMID: 10518567 Free PMC article.
-
Functions of autophagy in the tumor microenvironment and cancer metastasis.FEBS J. 2018 May;285(10):1751-1766. doi: 10.1111/febs.14388. Epub 2018 Feb 1. FEBS J. 2018. PMID: 29356327 Free PMC article. Review.
-
Dissecting the multifaceted roles of autophagy in cancer initiation, growth, and metastasis: from molecular mechanisms to therapeutic applications.Med Oncol. 2024 Jun 20;41(7):183. doi: 10.1007/s12032-024-02417-2. Med Oncol. 2024. PMID: 38902544 Review.
Cited by
-
Oxidized phospholipid damage signals as modulators of immunity.Open Biol. 2025 Jul;15(7):240391. doi: 10.1098/rsob.240391. Epub 2025 Jul 30. Open Biol. 2025. PMID: 40730233 Free PMC article. Review.
-
Lipoprotein (a)-Related Inflammatory Imbalance: A Novel Horizon for the Development of Atherosclerosis.Curr Atheroscler Rep. 2024 Aug;26(8):383-394. doi: 10.1007/s11883-024-01215-5. Epub 2024 Jun 15. Curr Atheroscler Rep. 2024. PMID: 38878139 Free PMC article. Review.
-
Rectal Cancer Tissue Lipidome Differs According to Response to Neoadjuvant Therapy.Int J Mol Sci. 2023 Jul 14;24(14):11479. doi: 10.3390/ijms241411479. Int J Mol Sci. 2023. PMID: 37511236 Free PMC article.
-
Atherosclerotic Oxidized Lipids Affect Formation and Biophysical Properties of Supported Lipid Bilayers and Simulated Membranes.J Phys Chem B. 2024 Nov 28;128(47):11694-11704. doi: 10.1021/acs.jpcb.4c05451. Epub 2024 Nov 18. J Phys Chem B. 2024. PMID: 39558641 Free PMC article.
-
Autophagy and Glycometabolic Reprograming in the Malignant Progression of Lung Cancer: A Review.Technol Cancer Res Treat. 2023 Jan-Dec;22:15330338231190545. doi: 10.1177/15330338231190545. Technol Cancer Res Treat. 2023. PMID: 37605558 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

