Bleeding by Bruton Tyrosine Kinase-Inhibitors: Dependency on Drug Type and Disease
- PMID: 33806595
- PMCID: PMC7961939
- DOI: 10.3390/cancers13051103
Bleeding by Bruton Tyrosine Kinase-Inhibitors: Dependency on Drug Type and Disease
Abstract
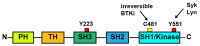
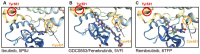
Bruton tyrosine kinase (Btk) is expressed in B-lymphocytes, myeloid cells and platelets, and Btk-inhibitors (BTKi) are used to treat patients with B-cell malignancies, developed against autoimmune diseases, have been proposed as novel antithrombotic drugs, and been tested in patients with severe COVID-19. However, mild bleeding is frequent in patients with B-cell malignancies treated with the irreversible BTKi ibrutinib and the recently approved 2nd generation BTKi acalabrutinib, zanubrutinib and tirabrutinib, and also in volunteers receiving in a phase-1 study the novel irreversible BTKi BI-705564. In contrast, no bleeding has been reported in clinical trials of other BTKi. These include the brain-penetrant irreversible tolebrutinib and evobrutinib (against multiple sclerosis), the irreversible branebrutinib, the reversible BMS-986142 and fenebrutinib (targeting rheumatoid arthritis and lupus erythematodes), and the reversible covalent rilzabrutinib (against pemphigus and immune thrombocytopenia). Remibrutinib, a novel highly selective covalent BTKi, is currently in clinical studies of autoimmune dermatological disorders. This review describes twelve BTKi approved or in clinical trials. By focusing on their pharmacological properties, targeted disease, bleeding side effects and actions on platelets it attempts to clarify the mechanisms underlying bleeding. Specific platelet function tests in blood might help to estimate the probability of bleeding of newly developed BTKi.
Keywords: Btk; Btk inhibitor; Tec; bleeding; covalent Btk inhibitor; hemorrhage; ibrutinib; platelet; reversible Btk inhibitor.
Conflict of interest statement
The authors have no conflict of interests and nothing to disclose.
Figures
References
-
- Rigg R.A., Aslan J.E., Healy L.D., Wallisch M., Thierheimer M.L., Loren C.P., Pang J., Hinds M.T., Gruber A., McCarty O.J. Oral administration of Bruton’s Tyrosine Kinase (Btk) inhibitors impairs GPVI-mediated platelet function. Am. J. Physiol. Cell Physiol. 2015;310:C373–C380. doi: 10.1152/ajpcell.00325.2015. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

