Classic Ketogenic Diet and Modified Atkins Diet in SLC2A1 Positive and Negative Patients with Suspected GLUT1 Deficiency Syndrome: A Single Center Analysis of 18 Cases
- PMID: 33806661
- PMCID: PMC8000344
- DOI: 10.3390/nu13030840
Classic Ketogenic Diet and Modified Atkins Diet in SLC2A1 Positive and Negative Patients with Suspected GLUT1 Deficiency Syndrome: A Single Center Analysis of 18 Cases
Abstract
Background: Glucose transporter type 1 deficiency syndrome (GLUT1DS) is caused by mutations in the SLC2A1 gene and produces seizures, neurodevelopmental impairment, and movement disorders. Ketogenic dietary therapies (KDT) are the gold standard treatment. Similar symptoms may appear in SLC2A1 negative patients. The purpose is to evaluate the effectiveness of KDT in children with GLUT1DS suspected SLC2A1 (+) and (-), side effects (SE), and the impact on patients nutritional status.
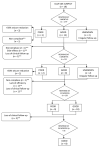
Methods: An observational descriptive study was conducted to describe 18 children (January 2009-August 2020). SLC2A1 analysis, seizures, movement disorder, anti-epileptic drugs (AEDS), anthropometry, SE, and laboratory assessment were monitored baseline and at 3, 6, 12, and 24 months after the onset of KDT.
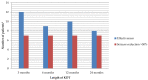
Results: 6/18 were SLC2A1(+) and 13/18 had seizures. In these groups, the age for debut of symptoms was higher. The mean time from debut to KDT onset was higher in SLC2A1(+). The modified Atkins diet (MAD) was used in 12 (5 SLC2A1(+)). Movement disorder improved (4/5), and a reduction in seizures >50% compared to baseline was achieved in more than half of the epileptic children throughout the follow-up. No differences in effectiveness were found according to the type of KDT. Early SE occurred in 33%. Long-term SE occurred in 10, 5, 7, and 5 children throughout the follow-up. The most frequent SE were constipation, hypercalciuria, and hyperlipidaemia. No differences in growth were found according to the SLC2A1 mutation or type of KDT.
Conclusions: CKD and MAD were effective for SLC2A1 positive and negative patients in our cohort. SE were frequent, but mild. Permanent monitoring should be made to identify SE and nutritional deficits.
Keywords: GLUT1 deficiency syndrome; SLC2A1 gene; ketogenic diet; movement disorder; pediatric epilepsy; refractory epilepsy.
Conflict of interest statement
Ruiz Herrero and González Gutiérrez-Solana have received honorarium for lectures and funding for travel from Nutricia. Cañedo Villarroya has received honorarium for lectures and funding for travel from Mead Johnson, Nestlé, Nutricia, Abbott, and Orphan. Puerta Macfarland has received honorarium for lectures from Nutricia. Pedrón Giner has served as consultant or received honorarium for lectures from Nutricia, Vitaflo, Nestlé, and Mead Jhonson; and has received funding for travel from Nutricia, Vitaflo, and Nestlé. García Alcolea and Gómez Fernández have no conflict of interest.
Figures

References
-
- De Vivo D.C., Trifiletti R.R., Jacobson R.I., Ronen G.M., Behmand R.A., Harik S.I. Defective glucose transport across the blood-brain barrier as a cause of persistent hypoglycorrhachia, seizures, and developmental delay. N. Engl. J. Med. 1991;325:703–709. doi: 10.1056/NEJM199109053251006. - DOI - PubMed
-
- Leen W.G., Klepper J., Verbeek M.M., Leferink M., Hofste T., Van Engelen B.G., Wevers R.A., Arthur T., Bahi-Buisson N., Ballhausen D., et al. Glucose transporter-1 deficiency syndrome: The expanding clinical and genetic spectrum of a treatable disorder. Brain. 2010;133:655–670. doi: 10.1093/brain/awp336. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

