The Multifunctional Sactipeptide Ruminococcin C1 Displays Potent Antibacterial Activity In Vivo as Well as Other Beneficial Properties for Human Health
- PMID: 33806791
- PMCID: PMC8005207
- DOI: 10.3390/ijms22063253
The Multifunctional Sactipeptide Ruminococcin C1 Displays Potent Antibacterial Activity In Vivo as Well as Other Beneficial Properties for Human Health
Abstract
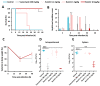
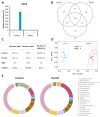
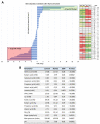
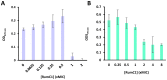
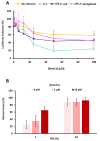
The world is on the verge of a major antibiotic crisis as the emergence of resistant bacteria is increasing, and very few novel molecules have been discovered since the 1960s. In this context, scientists have been exploring alternatives to conventional antibiotics, such as ribosomally synthesized and post-translationally modified peptides (RiPPs). Interestingly, the highly potent in vitro antibacterial activity and safety of ruminococcin C1, a recently discovered RiPP belonging to the sactipeptide subclass, has been demonstrated. The present results show that ruminococcin C1 is efficient at curing infection and at protecting challenged mice from Clostridium perfringens with a lower dose than the conventional antibiotic vancomycin. Moreover, antimicrobial peptide (AMP) is also effective against this pathogen in the complex microbial community of the gut environment, with a selective impact on a few bacterial genera, while maintaining a global homeostasis of the microbiome. In addition, ruminococcin C1 exhibits other biological activities that could be beneficial for human health, as well as other fields of applications. Overall, this study, by using an in vivo infection approach, confirms the antimicrobial clinical potential and highlights the multiple functional properties of ruminococcin C1, thus extending its therapeutic interest.
Keywords: Clostridium perfringens; RiPP; Ruminococcus gnavus E1; antibiotics; antimicrobial resistance; microbiome; peritonitis infection; sactipeptide.
Conflict of interest statement
The authors declare no conflict of interest and the sponsors had no role in the design, execution, interpretation, or writing of the study.
Figures
References
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

