Structure and Function of Ion Channels Regulating Sperm Motility-An Overview
- PMID: 33806823
- PMCID: PMC8004680
- DOI: 10.3390/ijms22063259
Structure and Function of Ion Channels Regulating Sperm Motility-An Overview
Abstract
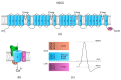
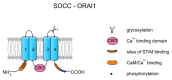
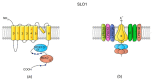
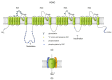
Sperm motility is linked to the activation of signaling pathways that trigger movement. These pathways are mainly dependent on Ca2+, which acts as a secondary messenger. The maintenance of adequate Ca2+ concentrations is possible thanks to proper concentrations of other ions, such as K+ and Na+, among others, that modulate plasma membrane potential and the intracellular pH. Like in every cell, ion homeostasis in spermatozoa is ensured by a vast spectrum of ion channels supported by the work of ion pumps and transporters. To achieve success in fertilization, sperm ion channels have to be sensitive to various external and internal factors. This sensitivity is provided by specific channel structures. In addition, novel sperm-specific channels or isoforms have been found with compositions that increase the chance of fertilization. Notably, the most significant sperm ion channel is the cation channel of sperm (CatSper), which is a sperm-specific Ca2+ channel required for the hyperactivation of sperm motility. The role of other ion channels in the spermatozoa, such as voltage-gated Ca2+ channels (VGCCs), Ca2+-activated Cl-channels (CaCCs), SLO K+ channels or voltage-gated H+ channels (VGHCs), is to ensure the activation and modulation of CatSper. As the activation of sperm motility differs among metazoa, different ion channels may participate; however, knowledge regarding these channels is still scarce. In the present review, the roles and structures of the most important known ion channels are described in regard to regulation of sperm motility in animals.
Keywords: calcium; chloride; ion channels; membrane channels; potassium; proton; sodium; sperm motility.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Ohtake H. Sperm-activating proteins obtained from the herring eggs. Fish Physiol. Biochem. 2003;28:199–202. doi: 10.1023/B:FISH.0000030527.87437.05. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

