Body Composition as a Predictor of Toxicity and Prognosis in Patients with Diffuse Large B-Cell Lymphoma Receiving R-CHOP Immunochemotherapy
- PMID: 33806839
- PMCID: PMC8025815
- DOI: 10.3390/curroncol28020126
Body Composition as a Predictor of Toxicity and Prognosis in Patients with Diffuse Large B-Cell Lymphoma Receiving R-CHOP Immunochemotherapy
Abstract
Background: Our study measured the body composition of Diffuse large B-cell lymphoma (DLBCL) patients receiving rituximab with cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP) regimen by computed tomographic (CT) and assessed their correlation with treatment-related toxicity and other adverse outcomes.
Methods: We retrospectively analyzed 201 DLBCL patients who underwent pre-treatment abdominal CT examination. CT images were used to assess body composition metrics at the third lumbar vertebrae including fat tissues and muscle. Based on the skeletal muscle area (SMA) and density (SMD), skeletal muscle index (SMI), skeletal muscle gauge (SMG = SMI × SMD) and lean body mass (LBM) were calculated. Also analyzed were the toxicity, adverse events and survival.
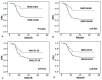
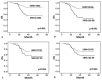
Results: We found that SMG, SMD, SMI and LBM were correlated with any grade 3-4 toxicity, dose reduction, hospitalization or termination of the treatment due to immunochemotherapy and worse survival. However, multivariate analysis demonstrated SMG [progression-free survival (PFS): hazard ratio (HR), 2.889; 95% CI, 1.401-5.959; p = 0.004; overall survival (OS): HR, 2.655; 95% CI, 1.218-5.787; p = 0.014] was the best predictor of poor prognosis.
Conclusions: SMG, SMD, SMI and LBM were identified as predictors of adverse reactions and poor survival. SMG was an innovative and valuable indicator of immunochemotherapy toxicity and other adverse outcomes. Additionally, it can be used to individualize antineoplastic drug dosing.
Keywords: diffuse large B-cell lymphoma; immunochemotherapy; sarcopenia; survival; toxicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Huang J.-J., Xia Y., Wang Y., Liu P.-P., Bi X.-W., Sun P., Lin T.-Y., Jiang W.-Q., Li Z.-M. A comparison of R-EPOCH and R-CHOP as a first-line regimen in de novo DLBCL patients with high Ki-67 expression in a single institution. Oncotarget. 2016;7:41242–41250. doi: 10.18632/oncotarget.9271. - DOI - PMC - PubMed
-
- Araie H., Sakamaki I., Matsuda Y., Tai K., Ikegaya S., Itoh K., Kishi S., Oiwa K., Okura M., Tasaki T., et al. 3A Comparison between R-THP-COP and R-CHOP Regimens for the Treatment of Diffuse Large B-cell Lymphoma in Old Patients: A Single-institution Analysis. Intern. Med. 2017;56:2407–2413. doi: 10.2169/internalmedicine.8291-16. - DOI - PMC - PubMed
-
- Intragumtornchai T., Sutheesophon J., Sutcharitchan P., Swasdikul D. A predictive model for life-threatening neutropenia and febrile neutropenia after the first course of CHOP chemotherapy in patients with aggressive non-Hodgkin’s lymphoma. Leuk. Lymphoma. 2000;37:351–360. doi: 10.3109/10428190009089435. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

