The Influence of Oxidative Stress on Serum Albumin Structure as a Carrier of Selected Diazaphenothiazine with Potential Anticancer Activity
- PMID: 33806875
- PMCID: PMC8005128
- DOI: 10.3390/ph14030285
The Influence of Oxidative Stress on Serum Albumin Structure as a Carrier of Selected Diazaphenothiazine with Potential Anticancer Activity
Abstract
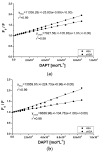
Albumin is one of the most important proteins in human blood. Among its multiple functions, drug binding is crucial in terms of drug distribution in human body. This protein undergoes many modifications that are certain to influence protein activity and affect its structure. One such reaction is albumin oxidation. Chloramine T is a strong oxidant. Solutions of human serum albumin, both non-modified and modified by chloramine T, were examined with the use of fluorescence, absorption and circular dichroism (CD) spectroscopy. 10H-3,6-diazaphenothiazine (DAPT) has anticancer activity and it has been studied for the first time in terms of binding with human serum albumin-its potential as a transporting protein. Using fluorescence spectroscopy, in the presence of dansylated amino acids, dansyl-l-glutamine (dGlu), dansyl-l-proline (dPro), DAPT binding with two main albumin sites-in subdomain IIA and IIIA-has been evaluated. Based on the conducted data, in order to measure the stability of DAPT complexes with human (HSA) and oxidized (oHSA) serum albumin, association constant (Ka) for ligand-HSA and ligand-oHSA complexes were calculated. It has been presumed that oxidation is not an important issue in terms of 10H-3,6-diazaphenothiazine binding to albumin. It means that the distribution of this substance is similar regardless of changes in albumin structure caused by oxidation, natural occurring in the organism.
Keywords: 10H-3,6-diazaphenothiazine; human serum albumin; oxidation; spectroscopic methods.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
-
- Peters T. All about Albumin: Biochemistry, Genetics and Medical Applications. Academic Press; San Diego, CA, USA: 1995.
-
- Otagiri M., Chuang V.T.G. Albumin in Medicine: Pathological and Clinical Applications. Springer; Singapore: 2016.
LinkOut - more resources
Full Text Sources
Other Literature Sources

