Proteomic Approaches to Dissect Host SUMOylation during Innate Antiviral Immune Responses
- PMID: 33806893
- PMCID: PMC8004987
- DOI: 10.3390/v13030528
Proteomic Approaches to Dissect Host SUMOylation during Innate Antiviral Immune Responses
Abstract
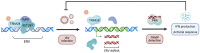
SUMOylation is a highly dynamic ubiquitin-like post-translational modification that is essential for cells to respond to and resolve various genotoxic and proteotoxic stresses. Virus infections also constitute a considerable stress scenario for cells, and recent research has started to uncover the diverse roles of SUMOylation in regulating virus replication, not least by impacting antiviral defenses. Here, we review some of the key findings of this virus-host interplay, and discuss the increasingly important contribution that large-scale, unbiased, proteomic methodologies are making to discoveries in this field. We highlight the latest proteomic technologies that have been specifically developed to understand SUMOylation dynamics in response to cellular stresses, and comment on how these techniques might be best applied to dissect the biology of SUMOylation during innate immunity. Furthermore, we showcase a selection of studies that have already used SUMO proteomics to reveal novel aspects of host innate defense against viruses, such as functional cross-talk between SUMO proteins and other ubiquitin-like modifiers, viral antagonism of SUMO-modified antiviral restriction factors, and an infection-triggered SUMO-switch that releases endogenous retroelement RNAs to stimulate antiviral interferon responses. Future research in this area has the potential to provide new and diverse mechanistic insights into host immune defenses.
Keywords: ISG15; SUMO; TRIM28; endogenous retroelements; influenza; innate immunity; interferon; proteomics; ubiquitin-like modification; virus infection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
An influenza virus-triggered SUMO switch orchestrates co-opted endogenous retroviruses to stimulate host antiviral immunity.Proc Natl Acad Sci U S A. 2019 Aug 27;116(35):17399-17408. doi: 10.1073/pnas.1907031116. Epub 2019 Aug 7. Proc Natl Acad Sci U S A. 2019. PMID: 31391303 Free PMC article.
-
Viruses, SUMO, and immunity: the interplay between viruses and the host SUMOylation system.J Neurovirol. 2021 Aug;27(4):531-541. doi: 10.1007/s13365-021-00995-9. Epub 2021 Aug 3. J Neurovirol. 2021. PMID: 34342851 Free PMC article. Review.
-
SUMOylation in Viral Replication and Antiviral Defense.Adv Sci (Weinh). 2022 Mar;9(7):e2104126. doi: 10.1002/advs.202104126. Epub 2022 Jan 21. Adv Sci (Weinh). 2022. PMID: 35060688 Free PMC article. Review.
-
Viral DNA Binding Protein SUMOylation Promotes PML Nuclear Body Localization Next to Viral Replication Centers.mBio. 2020 Mar 17;11(2):e00049-20. doi: 10.1128/mBio.00049-20. mBio. 2020. PMID: 32184235 Free PMC article.
-
The implication of SUMO in intrinsic and innate immunity.Cytokine Growth Factor Rev. 2016 Jun;29:3-16. doi: 10.1016/j.cytogfr.2016.04.003. Epub 2016 Apr 27. Cytokine Growth Factor Rev. 2016. PMID: 27157810 Review.
Cited by
-
Suppression of ACE2 SUMOylation protects against SARS-CoV-2 infection through TOLLIP-mediated selective autophagy.Nat Commun. 2022 Sep 3;13(1):5204. doi: 10.1038/s41467-022-32957-y. Nat Commun. 2022. PMID: 36057605 Free PMC article.
-
Human E3 ubiquitin ligases: accelerators and brakes for SARS-CoV-2 infection.Biochem Soc Trans. 2024 Oct 30;52(5):2009-2021. doi: 10.1042/BST20230324. Biochem Soc Trans. 2024. PMID: 39222407 Free PMC article. Review.
-
SUMOylation of Nuclear γ-Actin by SUMO2 supports DNA Damage Repair against Myocardial Ischemia-Reperfusion Injury.Int J Biol Sci. 2022 Jul 11;18(11):4595-4609. doi: 10.7150/ijbs.74407. eCollection 2022. Int J Biol Sci. 2022. PMID: 35864967 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

