Recent Developments on Semiconducting Polymer Nanoparticles as Smart Photo-Therapeutic Agents for Cancer Treatments-A Review
- PMID: 33806912
- PMCID: PMC8004612
- DOI: 10.3390/polym13060981
Recent Developments on Semiconducting Polymer Nanoparticles as Smart Photo-Therapeutic Agents for Cancer Treatments-A Review
Abstract
Semiconducting polymer nanoparticles (SPN) have been emerging as novel functional nano materials for phototherapy which includes PTT (photo-thermal therapy), PDT (photodynamic therapy), and their combination. Therefore, it is important to look into their recent developments and further explorations specifically in cancer treatment. Therefore, the present review describes novel semiconducting polymers at the nanoscale, along with their applications and limitations with a specific emphasis on future perspectives. Special focus is given on emerging and trending semiconducting polymeric nanoparticles in this review based on the research findings that have been published mostly within the last five years.
Keywords: applications; future scope; limitations; photo-therapy; semiconducting polymers.
Conflict of interest statement
The authors declare no conflict of interest. Additionally, the funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


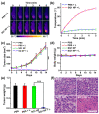
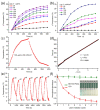


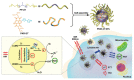

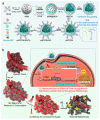
References
-
- Pinheiro P.S., Callahan K.E., Jones P.D., Morris C., Ransdell J.M., Kwon D., Brown C.P., Kobetz E.N. Liver cancer: A leading cause of cancer death in the United States and the role of the 1945–1965 birth cohort by ethnicity. JHEP Rep. 2019;1:162–169. doi: 10.1016/j.jhepr.2019.05.008. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

