Neonatal Mesenchymal Stem Cell Treatment Improves Myelination Impaired by Global Perinatal Asphyxia in Rats
- PMID: 33806988
- PMCID: PMC8004671
- DOI: 10.3390/ijms22063275
Neonatal Mesenchymal Stem Cell Treatment Improves Myelination Impaired by Global Perinatal Asphyxia in Rats
Abstract
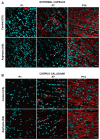
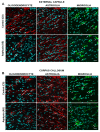
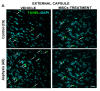
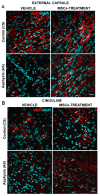
The effect of perinatal asphyxia (PA) on oligodendrocyte (OL), neuroinflammation, and cell viability was evaluated in telencephalon of rats at postnatal day (P)1, 7, and 14, a period characterized by a spur of neuronal networking, evaluating the effect of mesenchymal stem cell (MSCs)-treatment. The issue was investigated with a rat model of global PA, mimicking a clinical risk occurring under labor. PA was induced by immersing fetus-containing uterine horns into a water bath for 21 min (AS), using sibling-caesarean-delivered fetuses (CS) as controls. Two hours after delivery, AS and CS neonates were injected with either 5 μL of vehicle (10% plasma) or 5 × 104 MSCs into the lateral ventricle. Samples were assayed for myelin-basic protein (MBP) levels; Olig-1/Olig-2 transcriptional factors; Gglial phenotype; neuroinflammation, and delayed cell death. The main effects were observed at P7, including: (i) A decrease of MBP-immunoreactivity in external capsule, corpus callosum, cingulum, but not in fimbriae of hippocampus; (ii) an increase of Olig-1-mRNA levels; (iii) an increase of IL-6-mRNA, but not in protein levels; (iv) an increase in cell death, including OLs; and (v) MSCs treatment prevented the effect of PA on myelination, OLs number, and cell death. The present findings show that PA induces regional- and developmental-dependent changes on myelination and OLs maturation. Neonatal MSCs treatment improves survival of mature OLs and myelination in telencephalic white matter.
Keywords: apoptosis; hypomyelination; mesenchymal stem cells; myelination; neonatal asphyxia/ischemia; neuroinflammation; oligodendrocyte; periventricular leukomalacia; rat brain; telencephalon.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
-
- Volpe J.J. Neurology of the Newborn. WB Saunders; London, UK: 1995. Hypoxic-Ischemic encephalopathy: Neuropathology and pathogenesis; pp. 279–313.
-
- Herrera-Marschitz M., Morales P., Leyton L., Bustamante D., Klawitter V., Espina-Marchant P., Allende C., Lisboa F., Cunich G., Jara-Cavieres A., et al. Perinatal asphyxia: Current status and approaches towards neuroprotective strategies, with focus on sentinel proteins. Neurotox. Res. 2011;19:603–627. doi: 10.1007/s12640-010-9208-9. - DOI - PMC - PubMed
-
- Herrera-Marschitz M., Neira-Peña T., Rojas-Mancilla E., Espina-Marchant P., Esmar D., Pérez R., Muñoz V., Gutierrez-Hernandez M., Rivera B., Simola N., et al. Perinatal asphyxia: CNS development and deficits with delayed onset. Front. Neurosci. 2014;8:47. doi: 10.3389/fnins.2014.00047. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

