Whole-Body Pharmacokinetics and Physiologically Based Pharmacokinetic Model for Monomethyl Auristatin E (MMAE)
- PMID: 33807057
- PMCID: PMC8004929
- DOI: 10.3390/jcm10061332
Whole-Body Pharmacokinetics and Physiologically Based Pharmacokinetic Model for Monomethyl Auristatin E (MMAE)
Abstract
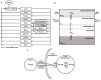
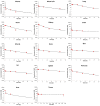
Monomethyl auristatin E (MMAE) is one of the most commonly used payloads for developing antibody-drug conjugates (ADC). However, limited studies have comprehensively evaluated the whole-body disposition of MMAE. Consequently, here, we have investigated the whole-body pharmacokinetics (PK) of MMAE in tumor-bearing mice. We show that while MMAE is rapidly eliminated from the plasma, it shows prolonged and extensive distribution in tissues, blood cells, and tumor. Highly perfused tissues (e.g., lung, kidney, heart, liver, and spleen) demonstrated tissue-to-plasma area under the concentration curve (AUC) ratios > 20, and poorly perfused tissues (e.g., fat, pancreas, skin, bone, and muscle) had ratios from 1.3 to 2.4. MMAE distribution was limited in the brain, and tumor had 8-fold higher exposure than plasma. A physiological-based pharmacokinetic (PBPK) model was developed to characterize the whole-body PK of MMAE, which accounted for perfusion/permeability-limited transfer of drug in the tissue, blood cell distribution of the drug, tissue/tumor retention of the drug, and plasma protein binding. The model was able to characterize the PK of MMAE in plasma, tissues, and tumor simultaneously, and model parameters were estimated with good precision. The MMAE PBPK model presented here can facilitate the development of a platform PBPK model for MMAE containing ADCs and help with their preclinical-to-clinical translation and clinical dose optimization.
Keywords: antibody–drug conjugate (ADC); biodistribution; monomethyl auristatin E (MMAE); physiological-based pharmacokinetic (PBPK) model; tissue pharmacokinetics (PK).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Kovtun Y.V., Audette C.A., Ye Y., Xie H., Ruberti M.F., Phinney S.J., Leece B.A., Chittenden T., Blättler W.A., Goldmacher V.S. Antibody-drug conjugates designed to eradicate tumors with homogeneous and heterogeneous expression of the target antigen. Cancer Res. 2006;66:3214–3221. doi: 10.1158/0008-5472.CAN-05-3973. - DOI - PubMed
-
- Adcetris Adcetris Assessment Report EMA/702390/2012. [(accessed on 9 September 2020)];2012 Jul 19; Available online: https://www.ema.europa.eu/en/documents/assessment-report/adcetris-epar-p....
-
- FDA The U.S. FDA Clinical Pharmacology and Biopharmaceutics Review for Brentuximab Vedotin. [(accessed on 9 September 2020)];2011 Available online: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/125388Orig1s000Cl....
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

