Immune Checkpoints Inhibitors and Chemotherapy as First-Line Treatment for Metastatic Urothelial Carcinoma: A Network Meta-Analysis of Randomized Phase III Clinical Trials
- PMID: 33807108
- PMCID: PMC8005008
- DOI: 10.3390/cancers13061484
Immune Checkpoints Inhibitors and Chemotherapy as First-Line Treatment for Metastatic Urothelial Carcinoma: A Network Meta-Analysis of Randomized Phase III Clinical Trials
Abstract
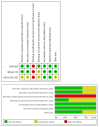
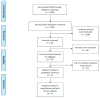
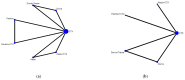
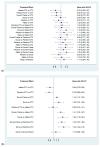
Immune checkpoints inhibitors (ICIs) were considered as second-line treatments in metastatic urothelial carcinoma (mUC) based on better survival benefit and safety profile than chemotherapy (CTX). We aimed to assess different ICIs regimens in the efficacy and safety for front-line treatments in mUC patients. A comprehensive literature search was performed and Phase II-III randomized controlled trials (RCTs) on ICIs for patients with mUC were included. The outcome was evaluated by overall survival (OS), progression of free survival (PFS), objective response rate (ORR), and grade 3-5 adverse events. Network meta-analysis was used to estimate the effect size. Surface under cumulative ranking curves (SUCRAs) were applied to rank the included treatments for each outcome. Results: The survival benefit of a single ICI was non-inferiority to chemotherapy (CTX). Although no superior effects were indicated, combination therapy (either ICIs plus CTX or ICIs plus ICIs) presented better OS compared with CTX alone. In terms of PFS, combination therapy produced a noticeable benefit over CTX. Regarding the SUCRA ranking, atezolizumab plus CTX was associated with the best ranking for OS and pembrolizumab plus CTX was the best in PFS. In terms of safety, a single ICI had better safety profile than CTX and combination therapy had a similar risk of grade 3-5 adverse events with CTX. Conclusions: Our NMA results revealed that combination therapy has better ranking compared with monotherapy in OS and acceptable AEs. ICIs alone present non-inferior OS but a lower incidence of AEs compared with CTX.
Keywords: chemotherapy; immune checkpoints inhibitors; network meta-analysis; urothelial carcinoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
The efficacy and safety of immune checkpoint inhibitors for patients with EGFR-mutated non-small cell lung cancer who progressed on EGFR tyrosine-kinase inhibitor therapy: A systematic review and network meta-analysis.Cancer Med. 2023 Sep;12(18):18516-18530. doi: 10.1002/cam4.6453. Epub 2023 Aug 16. Cancer Med. 2023. PMID: 37584242 Free PMC article. Review.
-
Selection of optimal first-line immuno-related therapy based on specific pathological characteristics for patients with advanced driver-gene wild-type non-small cell lung cancer: a systematic review and network meta-analysis.Ther Adv Med Oncol. 2021 May 29;13:17588359211018537. doi: 10.1177/17588359211018537. eCollection 2021. Ther Adv Med Oncol. 2021. PMID: 34104227 Free PMC article. Review.
-
The Differences in the Safety and Tolerability of Immune Checkpoint Inhibitors as Treatment for Non-Small Cell Lung Cancer and Melanoma: Network Meta-Analysis and Systematic Review.Front Pharmacol. 2019 Oct 24;10:1260. doi: 10.3389/fphar.2019.01260. eCollection 2019. Front Pharmacol. 2019. PMID: 31708783 Free PMC article.
-
Systematic Review and Network Meta-Analysis of Immune Checkpoint Inhibitors in Combination with Chemotherapy as a First-Line Therapy for Extensive-Stage Small Cell Carcinoma.Cancers (Basel). 2020 Dec 3;12(12):3629. doi: 10.3390/cancers12123629. Cancers (Basel). 2020. PMID: 33287455 Free PMC article.
-
Rational application of the first-line chemotherapy and immune checkpoint inhibitors in advanced nonsmall cell lung cancer: A meta-analysis.Cancer Med. 2019 Sep;8(11):5033-5046. doi: 10.1002/cam4.2407. Epub 2019 Jul 11. Cancer Med. 2019. PMID: 31297962 Free PMC article.
Cited by
-
Epigenetic modifiers synergize with immune-checkpoint blockade to enhance long-lasting antitumor efficacy.J Clin Invest. 2021 Aug 16;131(16):e151002. doi: 10.1172/JCI151002. J Clin Invest. 2021. PMID: 34396984 Free PMC article.
-
Systematic Literature Review (SLR) and Network Meta-Analysis (NMA) of First-Line Therapies (1L) for Locally Advanced/Metastatic Urothelial Carcinoma (la/mUC).Curr Oncol. 2023 Mar 26;30(4):3637-3647. doi: 10.3390/curroncol30040277. Curr Oncol. 2023. PMID: 37185390 Free PMC article.
-
The efficacy and safety of chemotherapy with or without anti-PD-1 for the first-line treatment of advanced urothelial carcinoma.Cancer Med. 2023 Dec;12(23):21129-21137. doi: 10.1002/cam4.6671. Epub 2023 Nov 21. Cancer Med. 2023. PMID: 37990780 Free PMC article.
-
Efficacy and Adverse Events of PD-1 Inhibitors in Patients With Advanced Urothelial Carcinoma From a Real-World Experience.Front Pharmacol. 2022 Mar 18;13:837499. doi: 10.3389/fphar.2022.837499. eCollection 2022. Front Pharmacol. 2022. PMID: 35370654 Free PMC article.
References
-
- Nakagawa T., Taguchi S., Kanatani A., Kawai T., Ikeda M., Urakami S., Matsumoto A., Komemushi Y., Miyakawa J., Yamada D., et al. Oncologic Outcome of Metastasectomy for Urothelial Carcinoma: Who Is the Best Candidate? Ann. Surg. Oncol. 2017;24:2794–2800. doi: 10.1245/s10434-017-5970-8. - DOI - PubMed
-
- Von Der Maase H., Sengelov L., Roberts J.T., Ricci S., Dogliotti L., Oliver T., Moore M.J., Zimmermann A., Arning M. Long-Term Survival Results of a Randomized Trial Comparing Gemcitabine Plus Cisplatin, With Methotrexate, Vinblastine, Doxorubicin, Plus Cisplatin in Patients With Bladder Cancer. J. Clin. Oncol. 2005;23:4602–4608. doi: 10.1200/JCO.2005.07.757. - DOI - PubMed
-
- Loehrer P.J., Einhorn L.H., Elson P.J., Crawford E.D., Kuebler P., Tannock I., Raghavan D., Stuart-Harris R., Sarosdy M.F., A Lowe B. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: A cooperative group study. J. Clin. Oncol. 1992;10:1066–1073. doi: 10.1200/JCO.1992.10.7.1066. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

