Assessment of Gastrointestinal Autonomic Dysfunction: Present and Future Perspectives
- PMID: 33807256
- PMCID: PMC8037288
- DOI: 10.3390/jcm10071392
Assessment of Gastrointestinal Autonomic Dysfunction: Present and Future Perspectives
Abstract
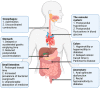
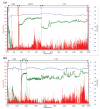
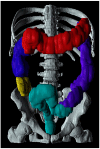
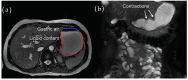
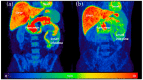
The autonomic nervous system delicately regulates the function of several target organs, including the gastrointestinal tract. Thus, nerve lesions or other nerve pathologies may cause autonomic dysfunction (AD). Some of the most common causes of AD are diabetes mellitus and α-synucleinopathies such as Parkinson's disease. Widespread dysmotility throughout the gastrointestinal tract is a common finding in AD, but no commercially available method exists for direct verification of enteric dysfunction. Thus, assessing segmental enteric physiological function is recommended to aid diagnostics and guide treatment. Several established assessment methods exist, but disadvantages such as lack of standardization, exposure to radiation, advanced data interpretation, or high cost, limit their utility. Emerging methods, including high-resolution colonic manometry, 3D-transit, advanced imaging methods, analysis of gut biopsies, and microbiota, may all assist in the evaluation of gastroenteropathy related to AD. This review provides an overview of established and emerging assessment methods of physiological function within the gut and assessment methods of autonomic neuropathy outside the gut, especially in regards to clinical performance, strengths, and limitations for each method.
Keywords: Parkinson’s disease; autonomic dysfunction; breath test; diabetes mellitus; gastrointestinal; imaging; investigations; manometry; motility.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the writing of the manuscript or in the decision to publish the results.
Figures
References
-
- Mathias C.J.B.R. Autonomic Failure. A Textbook of Clinical Disorders of the Autonomic Nervous System. 5th ed. University Press; Oxford, UK: 2013.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

