High Mobility Group Box 1 Promotes Lung Cancer Cell Migration and Motility via Regulation of Dynamin-Related Protein 1
- PMID: 33807275
- PMCID: PMC8036886
- DOI: 10.3390/ijms22073628
High Mobility Group Box 1 Promotes Lung Cancer Cell Migration and Motility via Regulation of Dynamin-Related Protein 1
Abstract
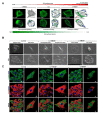
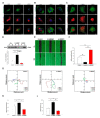
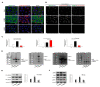
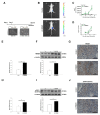
High mobility group box 1 (HMGB1) has been demonstrated to promote the migration and invasion of non-small cell lung cancer (NSCLC). However, the mechanism of action of HMGB1 in regulating tumor mobility remains unclear. Therefore, we aimed to investigate whether HMGB1 affects mitochondria distribution and regulates dynamin-related protein 1 (DRP1)-mediated lamellipodia/filopodia formation to promote NSCLC migration. The regulation of mitochondrial membrane tension, dynamics, polarization, fission process, and cytoskeletal rearrangements in lung cancer cells by HMGB1 was analyzed using confocal microscopy. The HMGB1-mediated regulation of DRP1 phosphorylation and colocalization was determined using immunostaining and co-immunoprecipitation assays. The tumorigenic potential of HMGB1 was assessed in vivo and further confirmed using NSCLC patient samples. Our results showed that HMGB1 increased the polarity and mobility of cells (mainly by regulating the cytoskeletal system actin and microtubule dynamics and distribution), promoted the formation of lamellipodia/filopodia, and enhanced the expression and phosphorylation of DRP1 in both the nucleus and cytoplasm. In addition, HMGB1 and DRP1 expressions were positively correlated and exhibited poor prognosis and survival in patients with lung cancer. Collectively, HMGB1 plays a key role in the formation of lamellipodia and filopodia by regulating cytoskeleton dynamics and DRP1 expression to promote lung cancer migration.
Keywords: cytoskeleton dynamics; dynamin related protein 1; high mobility group box 1; lamellipodia/filopodia; mitochondrial fission; non-small cell lung cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
- KMU-TC108A04-0/Kaohsiung Medical University Research Center Grant
- MOST 109-2314-B-030-002-MY2 and 108-2314-B-037-093-MY3/Ministry of Science and Technology, Taiwan
- KMUH108-8M10, and 108-8R14/Kaohsiung Medical University Chung-Ho Memorial Hospital
- CMU109-MF-106 and CMU109-S-37/China Medical University, Taiwan
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

