Alarming Cargo: The Role of Exosomes in Trauma-Induced Inflammation
- PMID: 33807302
- PMCID: PMC8065643
- DOI: 10.3390/biom11040522
Alarming Cargo: The Role of Exosomes in Trauma-Induced Inflammation
Abstract
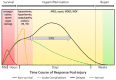
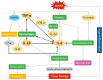
Severe polytraumatic injury initiates a robust immune response. Broad immune dysfunction in patients with such injuries has been well-documented; however, early biomarkers of immune dysfunction post-injury, which are critical for comprehensive intervention and can predict the clinical course of patients, have not been reported. Current circulating markers such as IL-6 and IL-10 are broad, non-specific, and lag behind the clinical course of patients. General blockade of the inflammatory response is detrimental to patients, as a certain degree of regulated inflammation is critical and necessary following trauma. Exosomes, small membrane-bound extracellular vesicles, found in a variety of biofluids, carry within them a complex functional cargo, comprised of coding and non-coding RNAs, proteins, and metabolites. Composition of circulating exosomal cargo is modulated by changes in the intra- and extracellular microenvironment, thereby serving as a homeostasis sensor. With its extensively documented involvement in immune regulation in multiple pathologies, study of exosomal cargo in polytrauma patients can provide critical insights on trauma-specific, temporal immune dysregulation, with tremendous potential to serve as unique biomarkers and therapeutic targets for timely and precise intervention.
Keywords: exosomes; extracellular vesicles; inflammation; intercellular communication; trauma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

