Complexity of Body Movements during Sleep in Children with Autism Spectrum Disorder
- PMID: 33807381
- PMCID: PMC8066562
- DOI: 10.3390/e23040418
Complexity of Body Movements during Sleep in Children with Autism Spectrum Disorder
Abstract
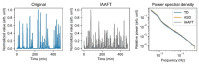
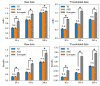
Recently, measuring the complexity of body movements during sleep has been proven as an objective biomarker of various psychiatric disorders. Although sleep problems are common in children with autism spectrum disorder (ASD) and might exacerbate ASD symptoms, their objectivity as a biomarker remains to be established. Therefore, details of body movement complexity during sleep as estimated by actigraphy were investigated in typically developing (TD) children and in children with ASD. Several complexity analyses were applied to raw and thresholded data of actigraphy from 17 TD children and 17 children with ASD. Determinism, irregularity and unpredictability, and long-range temporal correlation were examined respectively using the false nearest neighbor (FNN) algorithm, information-theoretic analyses, and detrended fluctuation analysis (DFA). Although the FNN algorithm did not reveal determinism in body movements, surrogate analyses identified the influence of nonlinear processes on the irregularity and long-range temporal correlation of body movements. Additionally, the irregularity and unpredictability of body movements measured by expanded sample entropy were significantly lower in ASD than in TD children up to two hours after sleep onset and at approximately six hours after sleep onset. This difference was found especially for the high-irregularity period. Through this study, we characterized details of the complexity of body movements during sleep and demonstrated the group difference of body movement complexity across TD children and children with ASD. Complexity analyses of body movements during sleep have provided valuable insights into sleep profiles. Body movement complexity might be useful as a biomarker for ASD.
Keywords: accelerometer; actigraphy; circadian rhythm disruption; detrended fluctuation analysis (DFA); entropy-based methods; expanded sample entropy (expSampEn); false nearest neighbors (FNN); information theory; insomnia; long-range temporal correlation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Atypical body movements during night in young children with autism spectrum disorder: a pilot study.Sci Rep. 2019 May 6;9(1):6999. doi: 10.1038/s41598-019-43397-y. Sci Rep. 2019. PMID: 31061424 Free PMC article.
-
Application of a novel actigraphy algorithm to detect movement and sleep/wake patterns in children with autism spectrum disorder.Sleep Med. 2020 Jul;71:28-34. doi: 10.1016/j.sleep.2020.02.020. Epub 2020 Mar 6. Sleep Med. 2020. PMID: 32454300 Free PMC article.
-
Sleep differences among children with autism spectrum disorders and typically developing peers: a meta-analysis.J Dev Behav Pediatr. 2015 Apr;36(3):166-77. doi: 10.1097/DBP.0000000000000140. J Dev Behav Pediatr. 2015. PMID: 25741949
-
Sleep in adults with Autism Spectrum Disorder: a systematic review and meta-analysis of subjective and objective studies.Sleep Med. 2020 Jan;65:113-120. doi: 10.1016/j.sleep.2019.07.019. Epub 2019 Aug 2. Sleep Med. 2020. PMID: 31739229
-
Assessment of Sleep in Children with Autism Spectrum Disorder.Children (Basel). 2017 Aug 8;4(8):72. doi: 10.3390/children4080072. Children (Basel). 2017. PMID: 28786962 Free PMC article. Review.
Cited by
-
General spectral characteristics of human activity and its inherent scale-free fluctuations.Sci Rep. 2024 Jan 31;14(1):2604. doi: 10.1038/s41598-024-52905-8. Sci Rep. 2024. PMID: 38297022 Free PMC article.
-
Parameter Analysis of Multiscale Two-Dimensional Fuzzy and Dispersion Entropy Measures Using Machine Learning Classification.Entropy (Basel). 2021 Oct 3;23(10):1303. doi: 10.3390/e23101303. Entropy (Basel). 2021. PMID: 34682027 Free PMC article.
-
Utility of complexity analysis in electroencephalography and electromyography for automated classification of sleep-wake states in mice.Sci Rep. 2025 Jan 24;15(1):3080. doi: 10.1038/s41598-024-74008-0. Sci Rep. 2025. PMID: 39856071 Free PMC article.
References
-
- Association A.P. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Publishing; Washington, DC, USA: 2013.
-
- Carmassi C., Palagini L., Caruso D., Masci I., Nobili L., Vita A., Dell’Osso L. Systematic review of sleep disturbances and circadian sleep desynchronization in autism spectrum disorder: Toward an integrative model of a self-reinforcing loop. Front. Psychiatry. 2019;10:366. doi: 10.3389/fpsyt.2019.00366. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

