Long-Term Iron Deficiency and Dietary Iron Excess Exacerbate Acute Dextran Sodium Sulphate-Induced Colitis and Are Associated with Significant Dysbiosis
- PMID: 33807459
- PMCID: PMC8037348
- DOI: 10.3390/ijms22073646
Long-Term Iron Deficiency and Dietary Iron Excess Exacerbate Acute Dextran Sodium Sulphate-Induced Colitis and Are Associated with Significant Dysbiosis
Abstract
Background: Oral iron supplementation causes gastrointestinal side effects. Short-term alterations in dietary iron exacerbate inflammation and alter the gut microbiota, in murine models of colitis. Patients typically take supplements for months. We investigated the impact of long-term changes in dietary iron on colitis and the microbiome in mice.
Methods: We fed mice chow containing differing levels of iron, reflecting deficient (100 ppm), normal (200 ppm), and supplemented (400 ppm) intake for up to 9 weeks, both in absence and presence of dextran sodium sulphate (DSS)-induced chronic colitis. We also induced acute colitis in mice taking these diets for 8 weeks. Impact was assessed (i) clinically and histologically, and (ii) by sequencing the V4 region of 16S rRNA.
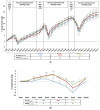
Results: In mice with long-term changes, the iron-deficient diet was associated with greater weight loss and histological inflammation in the acute colitis model. Chronic colitis was not influenced by altering dietary iron however there was a change in the microbiome in DSS-treated mice consuming 100 ppm and 400 ppm iron diets, and control mice consuming the 400 ppm iron diet. Proteobacteria levels increased significantly, and Bacteroidetes levels decreased, in the 400 ppm iron DSS group at day-63 compared to baseline.
Conclusions: Long-term dietary iron alterations affect gut microbiota signatures but do not exacerbate chronic colitis, however acute colitis is exacerbated by such dietary changes. More work is needed to understand the impact of iron supplementation on IBD. The change in the microbiome, in patients with colitis, may arise from the increased luminal iron and not simply from colitis.
Keywords: diet; fecal microbiota; inflammatory bowel disease; intestinal inflammation; iron.
Conflict of interest statement
The authors have declared that no competing interest exists. The funder had no role in study design, data collection and analysis, decision to publish, nor in preparation of the manuscript.
Figures




References
-
- Constante M., Fragoso G., Lupien-Meilleur J., Calvé A., Santos M.M. Iron Supplements Modulate Colon Microbiota Composition and Potentiate the Protective Effects of Probiotics in Dextran Sodium Sulfate-induced Colitis. Inflamm. Bowel Dis. 2017;23:753–766. doi: 10.1097/MIB.0000000000001089. - DOI - PubMed
-
- Manfred Wick W.P., Lehmann P. Clinical Aspects and Laboratory—Iron Metabolism. 6th ed. Springer; Vienna, Austria: 2011.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

