Peroral Clove Essential Oil Treatment Ameliorates Acute Campylobacteriosis-Results from a Preclinical Murine Intervention Study
- PMID: 33807493
- PMCID: PMC8066448
- DOI: 10.3390/microorganisms9040735
Peroral Clove Essential Oil Treatment Ameliorates Acute Campylobacteriosis-Results from a Preclinical Murine Intervention Study
Abstract
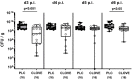
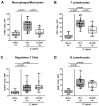
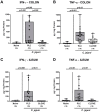
Campylobacter (C.) jejuni infections pose progressively emerging threats to human health worldwide. Given the rise in antibiotic resistance, antibiotics-independent options are required to fight campylobacteriosis. Since the health-beneficial effects of clove have been known for long, we here analyzed the antimicrobial and immune-modulatory effects of clove essential oil (EO) during acute experimental campylobacteriosis. Therefore, microbiota-depleted interleukin-10 deficient (IL-10-/-) mice were perorally infected with C. jejuni and treated with clove EO via drinking water starting on day 2 post-infection. On day 6 post-infection, lower small- and large-intestinal pathogen loads could be assessed in clove EO as compared to placebo treated mice. Although placebo mice suffered from severe campylobacteriosis as indicated by wasting and bloody diarrhea, clove EO treatment resulted in a better clinical outcome and in less severe colonic histopathological and apoptotic cell responses in C. jejuni infected mice. Furthermore, lower colonic numbers of macrophages, monocytes, and T lymphocytes were detected in mice from the verum versus the placebo cohort that were accompanied by lower intestinal, extra-intestinal, and even systemic proinflammatory cytokine concentrations. In conclusion, our preclinical intervention study provides first evidence that the natural compound clove EO constitutes a promising antibiotics-independent treatment option of acute campylobacteriosis in humans.
Keywords: Campylobacter jejuni; acute campylobacteriosis model; clove essential oil; enteropathogenic infection; eugenol; host-pathogen interaction; immune-modulatory effects; microbiota-depleted IL-10−/− mice; natural antibiotics-independent compounds; preclinical intervention study.
Conflict of interest statement
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures






Similar articles
-
Anti-Pathogenic and Immune-Modulatory Effects of Peroral Treatment with Cardamom Essential Oil in Acute Murine Campylobacteriosis.Microorganisms. 2021 Jan 14;9(1):169. doi: 10.3390/microorganisms9010169. Microorganisms. 2021. PMID: 33466708 Free PMC article.
-
Disease alleviating effects following prophylactic lemon and coriander essential oil treatment in mice with acute campylobacteriosis.Front Microbiol. 2023 Mar 29;14:1154407. doi: 10.3389/fmicb.2023.1154407. eCollection 2023. Front Microbiol. 2023. PMID: 37065112 Free PMC article.
-
Garlic Essential Oil as Promising Option for the Treatment of Acute Campylobacteriosis-Results from a Preclinical Placebo-Controlled Intervention Study.Microorganisms. 2021 May 25;9(6):1140. doi: 10.3390/microorganisms9061140. Microorganisms. 2021. PMID: 34070612 Free PMC article.
-
Novel Clinical Campylobacter jejuni Infection Models Based on Sensitization of Mice to Lipooligosaccharide, a Major Bacterial Factor Triggering Innate Immune Responses in Human Campylobacteriosis.Microorganisms. 2020 Mar 28;8(4):482. doi: 10.3390/microorganisms8040482. Microorganisms. 2020. PMID: 32231139 Free PMC article. Review.
-
Update on human Campylobacter jejuni infections.Curr Opin Gastroenterol. 2011 Jan;27(1):1-7. doi: 10.1097/MOG.0b013e3283413763. Curr Opin Gastroenterol. 2011. PMID: 21124212 Review.
Cited by
-
The glycosyltransferase ST3GAL2 is regulated by miR-615-3p in the intestinal tract of Campylobacter jejuni infected mice.Gut Pathog. 2021 Jun 28;13(1):42. doi: 10.1186/s13099-021-00437-1. Gut Pathog. 2021. PMID: 34183045 Free PMC article.
-
Disease-Alleviating Effects of Peroral Activated Charcoal Treatment in Acute Murine Campylobacteriosis.Microorganisms. 2021 Jun 30;9(7):1424. doi: 10.3390/microorganisms9071424. Microorganisms. 2021. PMID: 34209438 Free PMC article.
-
Molecular Targets in Campylobacter Infections.Biomolecules. 2023 Feb 22;13(3):409. doi: 10.3390/biom13030409. Biomolecules. 2023. PMID: 36979344 Free PMC article. Review.
-
Therapeutic Effects of Oral Application of Menthol and Extracts from Tormentil (Potentilla erecta), Raspberry Leaves (Rubus idaeus), and Loosestrife (Lythrum salicaria) during Acute Murine Campylobacteriosis.Pharmaceutics. 2023 Oct 1;15(10):2410. doi: 10.3390/pharmaceutics15102410. Pharmaceutics. 2023. PMID: 37896170 Free PMC article.
-
Treatment with the Probiotic Product Aviguard® Alleviates Inflammatory Responses during Campylobacter jejuni-Induced Acute Enterocolitis in Mice.Int J Mol Sci. 2021 Jun 22;22(13):6683. doi: 10.3390/ijms22136683. Int J Mol Sci. 2021. PMID: 34206478 Free PMC article.
References
-
- WHO World Health Organisation. Campylobacter. [(accessed on 4 June 2020)]; Available online: https://www.who.int/news-room/fact-sheets/detail/campylobacter.
-
- Heimesaat M.M., Backert S., Alter T., Bereswill S. Human Campylobacteriosis-A Serious Infectious Threat in a One Health Perspective. Curr. Top. Microbiol. Immunol. 2021;431:1–23. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

