Pharmacogenetics Approach for the Improvement of COVID-19 Treatment
- PMID: 33807592
- PMCID: PMC7998786
- DOI: 10.3390/v13030413
Pharmacogenetics Approach for the Improvement of COVID-19 Treatment
Abstract
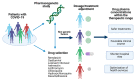
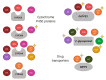
The treatment of coronavirus disease 2019 (COVID-19) has been a challenge. The efficacy of several drugs has been evaluated and variability in drug response has been observed. Pharmacogenetics could explain this variation and improve patients' outcomes with this complex disease; nevertheless, several disease-related issues must be carefully reviewed in the pharmacogenetic study of COVID-19 treatment. We aimed to describe the pharmacogenetic variants reported for drugs used for COVID-19 treatment (remdesivir, oseltamivir, lopinavir, ritonavir, azithromycin, chloroquine, hydroxychloroquine, ivermectin, and dexamethasone). In addition, other factors relevant to the design of pharmacogenetic studies were mentioned. Variants in CYP3A4, CYP3A5, CYP2C8, CY2D6, ABCB1, ABCC2, and SLCO1B1, among other variants, could be included in pharmacogenetic studies of COVID-19 treatment. Besides, nongenetic factors such as drug-drug interactions and inflammation should be considered in the search for personalized therapy of COVID-19.
Keywords: ABCB1; COVID-19; CYP2D6; CYP3A4; NR1I2; pharmacogenetics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- COVID-19 Treatment Guidelines. [(accessed on 19 December 2020)]; Available online: https://www.covid19treatmentguidelines.nih.gov/
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

