Recent Progress on Synthesis of N, N'-Chelate Organoboron Derivatives
- PMID: 33807680
- PMCID: PMC7961668
- DOI: 10.3390/molecules26051401
Recent Progress on Synthesis of N, N'-Chelate Organoboron Derivatives
Abstract
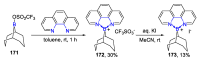
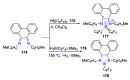
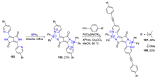
N,N'-chelate organoboron compounds have been successfully applied in bioimaging, organic light-emitting diodes (OLEDs), functional polymer, photocatalyst, electroluminescent (EL) devices, and other science and technology areas. However, the concise and efficient synthetic methods become more and more significant for material science, biomedical research, or other practical science. Here, we summarized the organoboron-N,N'-chelate derivatives and showed the different routes of their syntheses. Traditional methods to synthesize N,N'-chelate organoboron compounds were mainly using bidentate ligand containing nitrogen reacting with trivalent boron reagents. In this review, we described a series of bidentate ligands, such as bipyridine, 2-(pyridin-2-yl)-1H-indole, 2-(5-methyl-1H-pyrrol-2-yl)quinoline, N-(quinolin-8-yl)acetamide, 1,10-phenanthroline, and diketopyrrolopyrrole (DPP).
Keywords: N,N′-chelate; fluorescent materials; organoboron; tetracoordinated.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


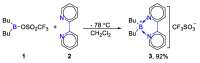
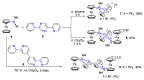
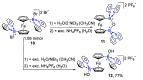
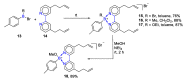
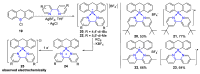
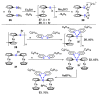

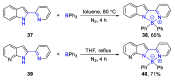

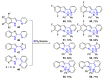




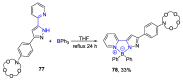
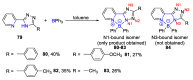


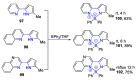




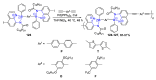


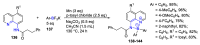



References
-
- Chi W., Chen J., Liu W., Wang C., Qi Q., Qiao Q., Tan T.M., Xiong K., Liu X., Kang K., et al. A General Descriptor DeltaE Enables the Quantitative Development of Luminescent Materials Based on Photoinduced Electron Transfer. J. Am. Chem. Soc. 2020;142:6777–6785. doi: 10.1021/jacs.0c01473. - DOI - PubMed
-
- Gartzia-Rivero L., Ray Leiva C., Sanchez-Carnerero E.M., Banuelos J., Moreno F., Maroto B.L., Garcia-Moreno I., Infantes L., Mendez B., Lopez-Arbeloa I., et al. Chiral Microneedles from an Achiral Bis(boron dipyrromethene): Spontaneous Mirror Symmetry Breaking Leading to a Promising Photoluminescent Organic Material. Langmuir. 2019;35:5021–5028. doi: 10.1021/acs.langmuir.9b00409. - DOI - PubMed
-
- Zhao Y., He S.L., Yang J.Y., Sun H., Shen X.F., Han X.G., Ni Z.H. Study on TICT emission of TPE-BODIPY derivatives mediated by methyl group on BODIPY. Opt. Mater. 2018;81:102–108. doi: 10.1016/j.optmat.2018.05.023. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

