GPR171 Activation Modulates Nociceptor Functions, Alleviating Pathologic Pain
- PMID: 33807709
- PMCID: PMC8001436
- DOI: 10.3390/biomedicines9030256
GPR171 Activation Modulates Nociceptor Functions, Alleviating Pathologic Pain
Abstract
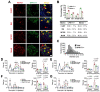
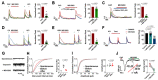
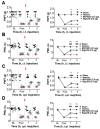
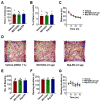
Modulation of the function of somatosensory neurons is an important analgesic strategy, requiring the proposal of novel molecular targets. Many G-protein-coupled receptors (GPRs) have been deorphanized, but the receptor locations, outcomes due to their activations, and their signal transductions remain to be elucidated, regarding the somatosensory nociceptor function. Here we report that GPR171, expressed in a nociceptor subpopulation, attenuated pain signals via Gi/o-coupled modulation of the activities of nociceptive ion channels when activated by its newly found ligands. Administration of its natural peptide ligand and a synthetic chemical ligand alleviated nociceptor-mediated acute pain aggravations and also relieved pathologic pain at nanomolar and micromolar ranges. This study suggests that functional alteration of the nociceptor neurons by GPR171 signaling results in pain alleviation and indicates that GPR171 is a promising molecular target for peripheral pain modulation.
Keywords: GPR171; TRP channel; analgesia; nociceptor; pain.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this article.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

