Analysis of Biofilm Formation on the Surface of Organic Mung Bean Seeds, Sprouts and in the Germination Environment
- PMID: 33807767
- PMCID: PMC7999400
- DOI: 10.3390/foods10030542
Analysis of Biofilm Formation on the Surface of Organic Mung Bean Seeds, Sprouts and in the Germination Environment
Abstract
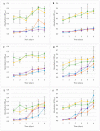
This study aimed to analyse the impact of sanitation methods on the formation of bacterial biofilms after disinfection and during the germination process of mung bean on seeds and in the germination environment. Moreover, the influence of Lactobacillus plantarum 299v on the growth of the tested pathogenic bacteria was evaluated. Three strains of Salmonella and E. coli were used for the study. The colony forming units (CFU), the crystal violet (CV), the LIVE/DEAD and the gram fluorescent staining, the light and the scanning electron microscopy (SEM) methods were used. The tested microorganisms survive in a small number. During germination after disinfection D2 (20 min H2O at 60 °C, then 15 min in a disinfecting mixture consisting of H2O, H2O2 and CH₃COOH), the biofilms grew most after day 2, but with the DP2 method (D2 + L. plantarum 299v during germination) after the fourth day. Depending on the method used, the second or fourth day could be a time for the introduction of an additional growth-limiting factor. Moreover, despite the use of seed disinfection, their germination environment could be favourable for the development of bacteria and, consequently, the formation of biofilms. The appropriate combination of seed disinfection methods and growth inhibition methods at the germination stage will lead to the complete elimination of the development of unwanted microflora and their biofilms.
Keywords: biofilm; mung bean; organic food; pathogen; probiotic; sprouts.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Mustar S., Nazaimoon W.M.W. The Effect of Sanitizers on the Native Microflora of Mung Bean Seeds (Vigna Radiata) J. Food Technol. 2010;8:234–238. doi: 10.3923/jftech.2010.234.238. - DOI
-
- Gabriel A.A. Microbial Quality of Chlorine Soaked Mung Bean Seeds and Sprouts. Food Sci. Technol. Res. 2005;11:95–100. doi: 10.3136/fstr.11.95. - DOI
-
- Zhang C., Lu Z., Li Y., Shang Y., Zhang G., Cao W. Reduction of Escherichia Coli O157:H7 and Salmonella Enteritidis on Mung Bean Seeds and Sprouts by Slightly Acidic Electrolyzed Water. Food Control. 2011;22:792–796. doi: 10.1016/j.foodcont.2010.11.018. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

