Synthesis of Fluorogenic Arylureas and Amides and Their Interaction with Amines: A Competition between Turn-on Fluorescence and Organic Radicals on the Way to a Smart Label for Fish Freshness
- PMID: 33807775
- PMCID: PMC7961427
- DOI: 10.3390/molecules26051404
Synthesis of Fluorogenic Arylureas and Amides and Their Interaction with Amines: A Competition between Turn-on Fluorescence and Organic Radicals on the Way to a Smart Label for Fish Freshness
Abstract
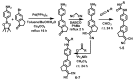
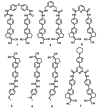
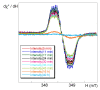
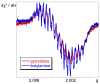
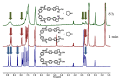
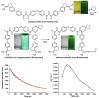
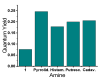
We describe the synthesis of fluorogenic arylureas and amides and their interaction with primary or secondary amines under air and light in organic-aqueous mixtures to give rise to a new class of persistent organic radicals, described on the basis of their electron paramagnetic resonance (EPR), as well as UV-vis, fluorescence, NMR, and quantum mechanics calculations, and their prospective use as multi-signal reporters in a smart label for fish freshness.
Keywords: arylureas; organic radicals; smart label; turn-on fluorescence; volatile amines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







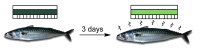
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

