SARS-CoV-2 Neutralizing Antibodies: A Network Meta-Analysis across Vaccines
- PMID: 33807818
- PMCID: PMC7999682
- DOI: 10.3390/vaccines9030227
SARS-CoV-2 Neutralizing Antibodies: A Network Meta-Analysis across Vaccines
Abstract
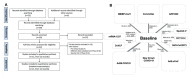
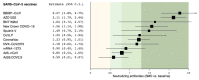
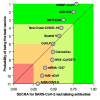
Background: There are no studies providing head-to-head comparison across SARS-CoV-2 vaccines. Therefore, we compared the efficacy of candidate vaccines in inducing neutralizing antibodies against SARS-CoV-2. Methods: A network meta-analysis was performed to compare the peak levels of SARS-CoV-2 neutralizing antibodies across candidate vaccines. Data were reported as standardized mean difference (SMD) since the outcome was assessed via different metrics and methods across the studies. Results: Data obtained from 836 healthy adult vaccine recipients were extracted from 11 studies. BBIBP-CorV, AZD1222, BNT162b2, New Crown COVID-19, and Sputnik V induced a very large effect on the level of neutralizing antibodies (SMD > 1.3); CoVLP, CoronaVac, NVX-CoV2373, and Ad5-nCoV induced a large effect (SMD > 0.8 to ≤1.3); and Ad26.COV2.S induced a medium effect (SMD > 0.5 to ≤0.8). BBIBP-CorV and AZD122 were more effective (p < 0.05) than Ad26.COV2.S, Ad5-nCoV, mRNA-1237, CoronaVac, NVX-CoV2373, CoVLP, and New Crown COVID-19; New Crown COVID-19 was more effective (p < 0.05) than Ad26.COV2.S, Ad5-nCoV, and mRNA-1237; CoronaVac was more effective (p < 0.05) than Ad26.COV2.S and Ad5-nCoV; and Sputnik V and BNT162b2 were more effective (p < 0.05) than Ad26.COV2.S. In recipients aged ≤60 years, AZD1222, BBIBP-CorV, and mRNA-1237 were the most effective candidate vaccines. Conclusion: All the candidate vaccines induced significant levels of SARS-CoV-2 neutralizing antibodies, but only AZD1222 and mRNA-1237 were certainly tested in patients aged ≥70 years. Compared with AZD1222, BNT162b and mRNA-1237 have the advantage that they can be quickly re-engineered to mimic new mutations of SARS-CoV-2.
Keywords: COVID-19; SARS-CoV-2; network meta-analysis; neutralizing antibodies; vaccine.
Conflict of interest statement
P.R. reports grants and personal fees from Boehringer Ingelheim, grants and personal fees from Novartis, personal fees from AstraZeneca, grants and personal fees from Chiesi Farmaceutici, grants and personal fees from Almirall, grants from Zambon, personal fees from Biofutura, personal fees from GlaxoSmithKline, and personal fees from Menarini, personal fees from Mundipharma. A.C. received grants from Menarini and AstraZeneca, and personal fees from Chiesi Farmaceutici. M.C. reports grants and personal fees from Boehringer Ingelheim, grants and personal fees from Novartis, personal fees from AstraZeneca, personal fees from Chiesi Farmaceutici, grants and personal fees from Almirall, personal fees from ABC Farmaceutici, personal fees from Edmond Pharma, grants and personal fees from Zambon, personal fees from Verona Pharma, personal fees from Ockham Biotech, personal fees from Biofutura, personal fees from GlaxoSmithKline, personal fees from Menarini, personal fees from Lallemand, personal fees from Mundipharma, and personal fees from Pfizer, outside the submitted work. L.C. has participated as advisor in scientific meetings under the sponsorship of Boehringer Ingelheim and Novartis; received nonfinancial support from AstraZeneca; a research grant partially funded by Chiesi Farmaceutici, Boehringer Ingelheim, Novartis, and Almirall; is or has been a consultant to ABC Farmaceutici, Edmond Pharma, Zambon, Verona Pharma, and Ockham Biotech; and his department was funded by Almirall, Boehringer Ingelheim, Chiesi Farmaceutici, Novartis, and Zambon. The authors declare no conflict of interest.
Figures

References
-
- Centers for Disease Control Prevention Ten great public health achievements—United States, 2001–2010. MMWR Morb. Mortal. Wkly Rep. 2011;60:619. - PubMed
-
- Gavi The Gavi COVAX AMC: An Investment Opportunity. [(accessed on 30 December 2020)]; Available online: http://www.gavi.org/covax-facility.
-
- World Health Organization Draft Landscape of COVID-19 Candidate Vaccines. [(accessed on 7 January 2021)]; Available online: http://www.dropbox.com/s/jiqqdl96g7qf3f1/20210106-Novel Coronavirus_Land....
-
- NCT04683224 A Study to Evaluate the Safety, Immunogenicity, and Efficacy of UB-612 COVID-19 Vaccine. [(accessed on 6 January 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04683224.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

