Two Siblings Homozygous for F508del-CFTR Have Varied Disease Phenotypes and Protein Biomarkers
- PMID: 33807880
- PMCID: PMC7961721
- DOI: 10.3390/ijms22052631
Two Siblings Homozygous for F508del-CFTR Have Varied Disease Phenotypes and Protein Biomarkers
Abstract
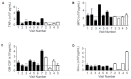
Two siblings with CF are homozygous for F508del (referred to as Subject A and Subject B). Despite having the same CFTR genotype and similar environment, these two subjects exhibited different disease phenotypes. We analyzed their medical records and CF Foundation Registry data and measured inflammatory protein mediators in their sputum samples. Then, we examined the longitudinal relationships between inflammatory markers and disease severity for each subject and compared between them. Subject A presented a more severe disease than Subject B. During the study period, Subject A had two pulmonary exacerbations (PEs) whereas Subject B had one mild PE. The forced expiratory volume in 1 s (FEV1, % predicted) values for Subject A were between 34-45% whereas for Subject B varied between 48-90%. Inflammatory protein mediators associated with neutrophils, Th1, Th2, and Th17 responses were elevated in sputum of Subject A compared with Subject B, and also in samples collected prior to and during PEs for both subjects. Neutrophilic elastase (NE) seemed to be the most informative biomarkers. The infectious burden between these two subjects was different.
Keywords: CFTR; F508del-CFTR; biomarkers; cystic fibrosis (CF); disease phenotypes; infection; inflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
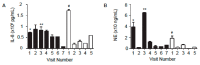


References
-
- Cystic Fibrosis Foundation [US] [(accessed on 30 July 2020)]; Available online: https://www.cff.org/What-is-CF/About-Cystic-Fibrosis/
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

