Cutin Synthesis in Developing, Field-Grown Apple Fruit Examined by External Feeding of Labelled Precursors
- PMID: 33807966
- PMCID: PMC8000455
- DOI: 10.3390/plants10030497
Cutin Synthesis in Developing, Field-Grown Apple Fruit Examined by External Feeding of Labelled Precursors
Abstract
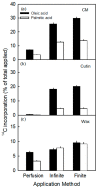
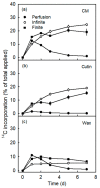
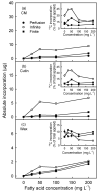
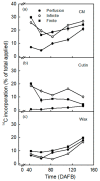
An intact skin is essential in high-quality apples. Ongoing deposition of cuticular material during fruit development may decrease microcracking. Our objective was to establish a system for quantifying cutin and wax deposition in developing apple fruit. Oleic acid (13C and 14C labelled) and palmitic acid (14C labelled) were fed to developing apples and the amounts incorporated in the cutin and wax fractions were quantified. The incorporation of 14C oleic acid (C18) was significantly higher than that of 14C palmitic acid (C16) and the incorporation in the cutin fraction exceeded that in the wax fraction. The amount of precursor incorporated in the cutin increased asymptotically with time, but the amount in the wax fraction remained about constant. Increasing the concentration of the precursor applied generally increased incorporation. Incorporation in the cutin fraction was high during early development (43 days after full bloom) and decreased towards maturity. Incorporation was higher from a dilute donor solution (infinite dose feeding) than from a donor solution subjected to drying (finite dose feeding) or from perfusion of the precursor by injection. Feeding the skin of a developing apple with oleic acid resulted in significant incorporation in the cutin fraction under both laboratory and field conditions.
Keywords: Malus × domestica; cuticle; cutin; epidermis; hypodermis; oleic acid; palmitic acid; wax.
Conflict of interest statement
There are no known competing interests that could influence the work reported in this paper.
Figures

References
-
- Knoche M., Lang A. Ongoing growth challenges fruit skin integrity. Crit. Rev. Plant Sci. 2017;36:190–215. doi: 10.1080/07352689.2017.1369333. - DOI
-
- Faust M., Shear C.B. Russeting of apples, an interpretive review. HortScience. 1972;7:233–235.
-
- Grimm E., Peschel S., Becker T., Knoche M. Stress and strain in the sweet cherry skin. J. Am. Soc. Hort. Sci. 2012;137:383–390. doi: 10.21273/JASHS.137.6.383. - DOI
-
- Kerstiens G. Cuticular water permeability and its physiological significance. J. Exp. Bot. 1996;47:1813–1832. doi: 10.1093/jxb/47.12.1813. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

